Novel in Vitro Method Reveals Drugs That Inhibit Organic Solute Transporter Alpha/Beta (OSTα/β)
- PMID: 30481467
- PMCID: PMC6465078
- DOI: 10.1021/acs.molpharmaceut.8b00966
Novel in Vitro Method Reveals Drugs That Inhibit Organic Solute Transporter Alpha/Beta (OSTα/β)
Abstract
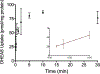
Drug interactions with the organic solute transporter alpha/beta (OSTα/β) are understudied even though OSTα/β is an important transporter that is expressed in multiple human tissues including the intestine, kidneys, and liver. In this study, an in vitro method to identify novel OSTα/β inhibitors was first developed using OSTα/β-overexpressing Flp-In 293 cells. Incubation conditions were optimized using previously reported OSTα/β inhibitors. A method including a 10 min preincubation step with the test compound was used to screen for OSTα/β inhibition by 77 structurally diverse compounds and fixed-dose combinations. Seven compounds and one fixed-dose combination (100 μM final concentration) inhibited OSTα/β-mediated dehydroepiandrosterone sulfate (DHEAS) uptake by >25%. Concentration-dependent OSTα/β inhibition was evaluated for all putative inhibitors (atorvastatin, ethinylestradiol, fidaxomicin, glycochenodeoxycholate, norgestimate, troglitazone, and troglitazone sulfate). Ethinylestradiol, fidaxomicin, and troglitazone sulfate yielded a clear concentration-inhibition response with IC50 values <200 μM. Among all tested compounds, there was no clear association between physicochemical properties, the severity of hepatotoxicity, and the degree of OSTα/β inhibition. This study utilized a novel in vitro method to identify OSTα/β inhibitors and, for the first time, provided IC50 values for OSTα/β inhibition. These data provide evidence that several drugs, some of which are associated with cholestatic drug-induced liver injury, may impair the function of the OSTα/β transporter.
Keywords: SLC51A/B; basolateral efflux; bile acid; cholestasis; drug-induced liver injury; inhibition.
Figures

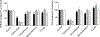
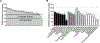

Similar articles
-
Organic solute transporter OSTα/β is overexpressed in nonalcoholic steatohepatitis and modulated by drugs associated with liver injury.Am J Physiol Gastrointest Liver Physiol. 2018 May 1;314(5):G597-G609. doi: 10.1152/ajpgi.00310.2017. Epub 2018 Feb 8. Am J Physiol Gastrointest Liver Physiol. 2018. PMID: 29420067 Free PMC article.
-
Role of Organic Solute Transporter Alpha/Beta in Hepatotoxic Bile Acid Transport and Drug Interactions.Toxicol Sci. 2020 Jul 1;176(1):34-35. doi: 10.1093/toxsci/kfaa052. Toxicol Sci. 2020. PMID: 32294204 Free PMC article.
-
Novel insights into the organic solute transporter alpha/beta, OSTα/β: From the bench to the bedside.Pharmacol Ther. 2020 Jul;211:107542. doi: 10.1016/j.pharmthera.2020.107542. Epub 2020 Apr 2. Pharmacol Ther. 2020. PMID: 32247663 Free PMC article. Review.
-
Organic solute transporter, OSTalpha-OSTbeta: its role in bile acid transport and cholestasis.Semin Liver Dis. 2010 May;30(2):178-85. doi: 10.1055/s-0030-1253226. Epub 2010 Apr 26. Semin Liver Dis. 2010. PMID: 20422499 Free PMC article. Review.
-
Upregulation of a basolateral FXR-dependent bile acid efflux transporter OSTalpha-OSTbeta in cholestasis in humans and rodents.Am J Physiol Gastrointest Liver Physiol. 2006 Jun;290(6):G1124-30. doi: 10.1152/ajpgi.00539.2005. Epub 2006 Jan 19. Am J Physiol Gastrointest Liver Physiol. 2006. PMID: 16423920
Cited by
-
Bile Acid Conjugation on Solid Nanoparticles Enhances ASBT-Mediated Endocytosis and Chylomicron Pathway but Weakens the Transcytosis by Inducing Transport Flow in a Cellular Negative Feedback Loop.Adv Sci (Weinh). 2022 Jul;9(21):e2201414. doi: 10.1002/advs.202201414. Epub 2022 Jun 2. Adv Sci (Weinh). 2022. PMID: 35652273 Free PMC article.
-
Effect of mTOR inhibitors on sodium taurocholate cotransporting polypeptide (NTCP) function in vitro.Front Pharmacol. 2023 Mar 22;14:1147495. doi: 10.3389/fphar.2023.1147495. eCollection 2023. Front Pharmacol. 2023. PMID: 37033614 Free PMC article.
-
Identification of Key Amino Acids that Impact Organic Solute Transporter α/β (OSTα/β).Mol Pharmacol. 2021 Dec;100(6):599-608. doi: 10.1124/molpharm.121.000345. Epub 2021 Oct 1. Mol Pharmacol. 2021. PMID: 34599072 Free PMC article.
-
Impact of Drug-Mediated Inhibition of Intestinal Transporters on Nutrient and Endogenous Substrate Disposition…an Afterthought?Pharmaceutics. 2024 Mar 24;16(4):447. doi: 10.3390/pharmaceutics16040447. Pharmaceutics. 2024. PMID: 38675109 Free PMC article. Review.
-
Role of Hepatocyte Transporters in Drug-Induced Liver Injury (DILI)-In Vitro Testing.Pharmaceutics. 2022 Dec 22;15(1):29. doi: 10.3390/pharmaceutics15010029. Pharmaceutics. 2022. PMID: 36678658 Free PMC article. Review.
References
-
- Brouwer KL; Keppler D; Hoffmaster KA; Bow DA; Cheng Y; Lai Y; Palm JE; Stieger B; Evers R In vitro methods to support transporter evaluation in drug discovery and development. Clin Pharmacol Ther 2013, 94, (1), 95–112. - PubMed
-
- Seward DJ; Koh AS; Boyer JL; Ballatori N Functional complementation between a novel mammalian polygenic transport complex and an evolutionarily ancient organic solute transporter, OSTalpha-OSTbeta. J Biol Chem 2003, 278, (30), 27473–82. - PubMed
-
- Ballatori N; Christian WV; Lee JY; Dawson PA; Soroka CJ; Boyer JL; Madejczyk MS; Li N OSTalpha-OSTbeta: a major basolateral bile acid and steroid transporter in human intestinal, renal, and biliary epithelia. Hepatology 2005, 42, (6), 1270–9. - PubMed
-
- Uhlen M; Fagerberg L; Hallstrom BM; Lindskog C; Oksvold P; Mardinoglu A; Sivertsson A; Kampf C; Sjostedt E; Asplund A; Olsson I; Edlund K; Lundberg E; Navani S; Szigyarto CA; Odeberg J; Djureinovic D; Takanen JO; Hober S; Alm T; Edqvist PH; Berling H; Tegel H; Mulder J; Rockberg J; Nilsson P; Schwenk JM; Hamsten M; von Feilitzen K; Forsberg M; Persson L; Johansson F; Zwahlen M; von Heijne G; Nielsen J; Ponten F Proteomics. Tissue-based map of the human proteome. Science 2015, 347, (6220), 1260419. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

