Chronic Intermittent Ethanol Exposure Selectively Increases Synaptic Excitability in the Ventral Domain of the Rat Hippocampus
- PMID: 30481568
- PMCID: PMC6658135
- DOI: 10.1016/j.neuroscience.2018.11.028
Chronic Intermittent Ethanol Exposure Selectively Increases Synaptic Excitability in the Ventral Domain of the Rat Hippocampus
Abstract
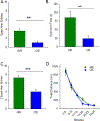
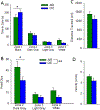
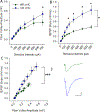
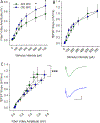
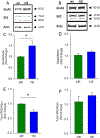
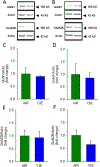
Many studies have implicated hippocampal dysregulation in the pathophysiology of alcohol use disorder (AUD). However, over the past twenty years, a growing body of evidence has revealed distinct functional roles of the dorsal (dHC) and ventral (vHC) hippocampal subregions, with the dHC being primarily involved in spatial learning and memory and the vHC regulating anxiety- and depressive-like behaviors. Notably, to our knowledge, no rodent studies have examined the effects of chronic ethanol exposure on synaptic transmission along the dorsal/ventral axis. To that end, we examined the effects of the chronic intermittent ethanol vapor exposure (CIE) model of AUD on dHC and vHC synaptic excitability. Adult male Long-Evans rats were exposed to CIE or AIR for 10 days (12 h/day; targeting blood ethanol levels of 175-225 mg%) and recordings were made 24 h into withdrawal. As expected, this protocol increased anxiety-like behaviors on the elevated plus-maze and successive alleys test. Extracellular recordings revealed marked CIE-associated increases in synaptic excitation in the CA1 region that were exclusively restricted to the ventral domain of the hippocampus. Western blot analysis of synaptoneurosomal fractions revealed that the expression of two proteins that regulate synaptic strength, GluA2 and SK2, were dysregulated in the vHC, but not the dHC, following CIE. Together, these findings suggest that the ventral CA1 region may be particularly sensitive to the maladaptive effects of chronic ethanol exposure and provide new insight into some of the neural substrates that may contribute to the negative affective state that develops during withdrawal.
Keywords: SK Calcium-activated potassium channels; anxiety; chronic intermittent ethanol; dorsal hippocampus; ventral hippocampus; withdrawal.
Copyright © 2018 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
Chronic intermittent ethanol promotes ventral subiculum hyperexcitability via increases in extrinsic basolateral amygdala input and local network activity.Sci Rep. 2021 Apr 22;11(1):8749. doi: 10.1038/s41598-021-87899-0. Sci Rep. 2021. PMID: 33888757 Free PMC article.
-
Small conductance calcium-activated potassium type 2 channels regulate alcohol-associated plasticity of glutamatergic synapses.Biol Psychiatry. 2011 Apr 1;69(7):625-32. doi: 10.1016/j.biopsych.2010.09.025. Epub 2010 Nov 5. Biol Psychiatry. 2011. PMID: 21056409 Free PMC article.
-
Chronic intermittent ethanol exposure enhances NMDA-receptor-mediated synaptic responses and NMDA receptor expression in hippocampal CA1 region.Brain Res. 2005 Jun 28;1048(1-2):69-79. doi: 10.1016/j.brainres.2005.04.041. Brain Res. 2005. PMID: 15919065
-
Alcohol impairs hippocampal function: From NMDA receptor synaptic transmission to mitochondrial function.Drug Alcohol Depend. 2019 Dec 1;205:107628. doi: 10.1016/j.drugalcdep.2019.107628. Epub 2019 Oct 17. Drug Alcohol Depend. 2019. PMID: 31683244 Review.
-
Alcohol and adult hippocampal neurogenesis: promiscuous drug, wanton effects.Prog Neuropsychopharmacol Biol Psychiatry. 2014 Oct 3;54:103-13. doi: 10.1016/j.pnpbp.2014.05.003. Epub 2014 May 17. Prog Neuropsychopharmacol Biol Psychiatry. 2014. PMID: 24842804 Free PMC article. Review.
Cited by
-
Effect of Alcohol on Hippocampal-Dependent Plasticity and Behavior: Role of Glutamatergic Synaptic Transmission.Front Behav Neurosci. 2020 Jan 24;13:288. doi: 10.3389/fnbeh.2019.00288. eCollection 2019. Front Behav Neurosci. 2020. PMID: 32038190 Free PMC article. Review.
-
Modulating depressive-like behaviors, memory impairment, and oxidative stress in chronic stress rat model using visible light therapy.Sci Rep. 2025 Jul 17;15(1):25943. doi: 10.1038/s41598-025-10854-w. Sci Rep. 2025. PMID: 40676115 Free PMC article.
-
Chronic intermittent ethanol promotes ventral subiculum hyperexcitability via increases in extrinsic basolateral amygdala input and local network activity.Sci Rep. 2021 Apr 22;11(1):8749. doi: 10.1038/s41598-021-87899-0. Sci Rep. 2021. PMID: 33888757 Free PMC article.
-
Repeated ethanol exposure and withdrawal alters ACE2 expression in discrete brain regions: Implications for SARS-CoV-2 infection.bioRxiv [Preprint]. 2022 Mar 29:2022.03.29.486282. doi: 10.1101/2022.03.29.486282. bioRxiv. 2022. Update in: Alcohol Clin Exp Res (Hoboken). 2023 Feb;47(2):219-239. doi: 10.1111/acer.15000. PMID: 35378747 Free PMC article. Updated. Preprint.
-
A History of Low-Dose Ethanol Shifts the Role of Ventral Hippocampus during Reward Seeking in Male Mice.eNeuro. 2023 May 16;10(5):ENEURO.0087-23.2023. doi: 10.1523/ENEURO.0087-23.2023. Print 2023 May. eNeuro. 2023. PMID: 37156611 Free PMC article.
References
-
- Babiec WE, Jami SA, Guglietta R, Chen PB, O’Dell TJ, 2017. Differential Regulation of NMDA Receptor-Mediated Transmission by SK Channels Underlies Dorsal-Ventral Differences in Dynamics of Schaffer Collateral Synaptic Function. J NeurosciJ Neurosci 37, 1950–1964. https://doi.org/0.1523/JNEUROSCI.3196-16.2017 - PMC - PubMed
-
- Bailey KR, Crawley JN, 2009. Anxiety-related behaviors in mice.In: Methods of Behavioral Analysis in Neuroscience, vol. 2 (Buccafusco JJ ed) CRC Press.
Publication types
MeSH terms
Substances
Grants and funding
- P50 AA026117/AA/NIAAA NIH HHS/United States
- F31 AA020439/AA/NIAAA NIH HHS/United States
- T32 AA007565/AA/NIAAA NIH HHS/United States
- AA26455 /NH/NIH HHS/United States
- R01 AA026551/AA/NIAAA NIH HHS/United States
- R21 AA026455/AA/NIAAA NIH HHS/United States
- T32 DA041349/DA/NIDA NIH HHS/United States
- R37 AA017531/AA/NIAAA NIH HHS/United States
- AA26117 /NH/NIH HHS/United States
- AA17531 /NH/NIH HHS/United States
- AA7565 /NH/NIH HHS/United States
- F31 AA025819/AA/NIAAA NIH HHS/United States
- AA25819 /NH/NIH HHS/United States
- AA26551 /NH/NIH HHS/United States
- R01 AA017531/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

