The role of posttranslational modifications of α-synuclein and LRRK2 in Parkinson's disease: Potential contributions of environmental factors
- PMID: 30481588
- PMCID: PMC6534484
- DOI: 10.1016/j.bbadis.2018.11.017
The role of posttranslational modifications of α-synuclein and LRRK2 in Parkinson's disease: Potential contributions of environmental factors
Abstract
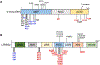
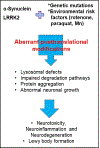
Parkinson's disease (PD) is the second most common neurodegenerative disease after Alzheimer's disease (AD), and the most prevalent movement disorder. PD is characterized by dopaminergic neurodegeneration in the substantia nigra, but its etiology has yet to be established. Among several genetic variants contributing to PD pathogenesis, α-synuclein and leucine-rich repeat kinase (LRRK2) are widely associated with neuropathological phenotypes in familial and sporadic PD. α-Synuclein and LRRK2 found in Lewy bodies, a pathogenic hallmark of PD, are often posttranslationally modified. As posttranslational modifications (PTMs) are key processes in regulating the stability, localization, and function of proteins, PTMs have emerged as important modulators of α-synuclein and LRRK2 pathology. Aberrant PTMs altering phosphorylation, ubiquitination, nitration and truncation of these proteins promote PD pathogenesis, while other PTMs such as sumoylation may be protective. Although the causes of many aberrant PTMs are unknown, environmental risk factors may contribute to their aberrancy. Environmental toxicants such as rotenone and paraquat have been shown to interact with these proteins and promote their abnormal PTMs. Notably, manganese (Mn) exposure leads to a PD-like neurological disorder referred to as manganism-and induces pathogenic PTMs of α-synuclein and LRRK2. In this review, we highlight the role of PTMs of α-synuclein and LRRK2 in PD pathogenesis and discuss the impact of environmental risk factors on their aberrancy.
Keywords: LRRK2; Manganese; Parkinson's disease; Posttranslational modifications; α-Synuclein.
Copyright © 2018. Published by Elsevier B.V.
Conflict of interest statement
Conflict of Interest
The authors declare no conflict of interest.
Figures
Similar articles
-
Interaction of LRRK2 and α-Synuclein in Parkinson's Disease.Adv Neurobiol. 2017;14:209-226. doi: 10.1007/978-3-319-49969-7_11. Adv Neurobiol. 2017. PMID: 28353286 Review.
-
Parkinson's Disease-Associated LRRK2 Interferes with Astrocyte-Mediated Alpha-Synuclein Clearance.Mol Neurobiol. 2021 Jul;58(7):3119-3140. doi: 10.1007/s12035-021-02327-8. Epub 2021 Feb 24. Mol Neurobiol. 2021. PMID: 33629273 Free PMC article.
-
Abundant non-inclusion α-synuclein pathology in Lewy body-negative LRRK2-mutant cases.Acta Neuropathol. 2025 May 2;149(1):41. doi: 10.1007/s00401-025-02871-w. Acta Neuropathol. 2025. PMID: 40314782 Free PMC article.
-
Analysis of macroautophagy related proteins in G2019S LRRK2 Parkinson's disease brains with Lewy body pathology.Brain Res. 2018 Dec 15;1701:75-84. doi: 10.1016/j.brainres.2018.07.023. Epub 2018 Jul 25. Brain Res. 2018. PMID: 30055128 Free PMC article.
-
α-Synuclein, leucine-rich repeat kinase-2, and manganese in the pathogenesis of Parkinson disease.Neurotoxicology. 2011 Oct;32(5):622-9. doi: 10.1016/j.neuro.2011.01.003. Epub 2011 Jan 14. Neurotoxicology. 2011. PMID: 21238487 Free PMC article. Review.
Cited by
-
LRRK2 kinase plays a critical role in manganese-induced inflammation and apoptosis in microglia.PLoS One. 2019 Jan 15;14(1):e0210248. doi: 10.1371/journal.pone.0210248. eCollection 2019. PLoS One. 2019. PMID: 30645642 Free PMC article.
-
Astrocytic Oxidative/Nitrosative Stress Contributes to Parkinson's Disease Pathogenesis: The Dual Role of Reactive Astrocytes.Antioxidants (Basel). 2019 Aug 1;8(8):265. doi: 10.3390/antiox8080265. Antioxidants (Basel). 2019. PMID: 31374936 Free PMC article. Review.
-
Neurotoxicity mechanisms of manganese in the central nervous system.Adv Neurotoxicol. 2021;5:215-238. doi: 10.1016/bs.ant.2020.11.003. Epub 2021 Jan 27. Adv Neurotoxicol. 2021. PMID: 34263091 Free PMC article. No abstract available.
-
Validation of differentially expressed brain-enriched microRNAs in the plasma of PD patients.Ann Clin Transl Neurol. 2020 Sep;7(9):1594-1607. doi: 10.1002/acn3.51146. Epub 2020 Aug 29. Ann Clin Transl Neurol. 2020. PMID: 32860338 Free PMC article.
-
Nonclinical safety evaluation, pharmacokinetics, and target engagement of Lu AF82422, a monoclonal IgG1 antibody against alpha-synuclein in development for treatment of synucleinopathies.MAbs. 2021 Jan-Dec;13(1):1994690. doi: 10.1080/19420862.2021.1994690. MAbs. 2021. PMID: 34709986 Free PMC article.
References
-
- Ozansoy M and Basak AN, The central theme of Parkinson’s disease: alpha-synuclein. Mol Neurobiol, 2013. 47(2): p. 460–5. - PubMed
-
- Barrett PJ and Timothy Greenamyre J, Post-translational modification of alpha-synuclein in Parkinson’s disease. Brain Res, 2015. 1628(Pt B): p. 247–253. - PubMed
-
- Sheng Z, et al., Ser1292 autophosphorylation is an indicator of LRRK2 kinase activity and contributes to the cellular effects of PD mutations. Sci Transl Med, 2012. 4(164): p. 164ra161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

