Breast Cancer Chemo-immunotherapy through Liposomal Delivery of an Immunogenic Cell Death Stimulus Plus Interference in the IDO-1 Pathway
- PMID: 30481959
- PMCID: PMC6262474
- DOI: 10.1021/acsnano.8b05189
Breast Cancer Chemo-immunotherapy through Liposomal Delivery of an Immunogenic Cell Death Stimulus Plus Interference in the IDO-1 Pathway
Retraction in
-
Retraction of "Breast Cancer Chemo-immunotherapy through Liposomal Delivery of an Immunogenic Cell Death Stimulus Plus Interference in the IDO-1 Pathway".ACS Nano. 2021 Jun 22;15(6):10735. doi: 10.1021/acsnano.0c08653. Epub 2021 May 21. ACS Nano. 2021. PMID: 34018715 Free PMC article. No abstract available.
Abstract
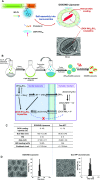
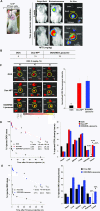
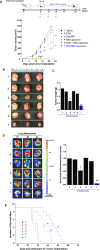
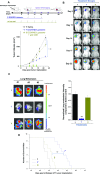
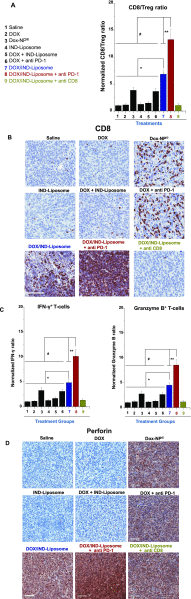
Immunotherapy provides the best approach to reduce the high mortality of metastatic breast cancer (BC). We demonstrate a chemo-immunotherapy approach, which utilizes a liposomal carrier to simultaneously trigger immunogenic cell death (ICD) as well as interfere in the regionally overexpressed immunosuppressive effect of indoleamine 2,3-dioxygenase (IDO-1) at the BC tumor site. The liposome was constructed by self-assembly of a phospholipid-conjugated prodrug, indoximod (IND), which inhibits the IDO-1 pathway, followed by the remote loading of the ICD-inducing chemo drug, doxorubicin (DOX). Intravenous injection of the encapsulated two-drug combination dramatically improved the pharmacokinetics and tumor drug concentrations of DOX and IND in an orthotopic 4T1 tumor model in syngeneic mice. Delivery of a threshold ICD stimulus resulted in the uptake of dying BC cells by dendritic cells, tumor antigen presentation and the activation/recruitment of naı̈ve T-cells. The subsequent activation of perforin- and IFN-γ releasing cytotoxic T-cells induced robust tumor cell killing at the primary as well as metastatic tumor sites. Immune phenotyping of the tumor tissues confirmed the recruitment of CD8+ cytotoxic T lymphocytes (CTLs), disappearance of Tregs, and an increase in CD8+/FOXP3+ T-cell ratios. Not only does the DOX/IND-Liposome provide a synergistic antitumor response that is superior to a DOX-only liposome, but it also demonstrated that the carrier could be effectively combined with PD-1 blocking antibodies to eradicate lung metastases. All considered, an innovative nano-enabled approach has been established to allow deliberate use of ICD to switch an immune deplete to an immune replete BC microenvironment, allowing further boosting of the response by coadministered IDO inhibitors or immune checkpoint blocking antibodies.
Keywords: breast cancer; chemo-immunotherapy; doxorubicin; dual-delivery liposome; immune checkpoint; immunogenic cell death; indoleamine 2,3-dioxygenase.
Conflict of interest statement
The authors declare the following competing financial interest(s): Andre E. Nel and Huan Meng are co-founders and equity holders in Westwood Biosciences Inc. The remaining authors declare no conflict of interest.
Figures




Similar articles
-
Dual functional immunostimulatory polymeric prodrug carrier with pendent indoximod for enhanced cancer immunochemotherapy.Acta Biomater. 2019 May;90:300-313. doi: 10.1016/j.actbio.2019.03.048. Epub 2019 Mar 28. Acta Biomater. 2019. PMID: 30930305 Free PMC article.
-
Liposomal Delivery of Mitoxantrone and a Cholesteryl Indoximod Prodrug Provides Effective Chemo-immunotherapy in Multiple Solid Tumors.ACS Nano. 2020 Oct 27;14(10):13343-13366. doi: 10.1021/acsnano.0c05194. Epub 2020 Sep 25. ACS Nano. 2020. PMID: 32940463 Free PMC article.
-
Tumor Microenvironment-Triggered Charge Reversal Polymetformin-Based Nanosystem Co-Delivered Doxorubicin and IL-12 Cytokine Gene for Chemo-Gene Combination Therapy on Metastatic Breast Cancer.ACS Appl Mater Interfaces. 2020 Oct 14;12(41):45873-45890. doi: 10.1021/acsami.0c14405. Epub 2020 Sep 29. ACS Appl Mater Interfaces. 2020. PMID: 32924511
-
Indoleamine 2,3-Dioxygenase (IDO) Inhibition as a Strategy to Augment Cancer Immunotherapy.BioDrugs. 2018 Aug;32(4):311-317. doi: 10.1007/s40259-018-0291-4. BioDrugs. 2018. PMID: 29980987 Review.
-
Chemo-Immunotherapy: Role of Indoleamine 2,3-Dioxygenase in Defining Immunogenic Versus Tolerogenic Cell Death in the Tumor Microenvironment.Adv Exp Med Biol. 2017;1036:91-104. doi: 10.1007/978-3-319-67577-0_7. Adv Exp Med Biol. 2017. PMID: 29275467 Free PMC article. Review.
Cited by
-
Nanomaterials: leading immunogenic cell death-based cancer therapies.Front Immunol. 2024 Aug 9;15:1447817. doi: 10.3389/fimmu.2024.1447817. eCollection 2024. Front Immunol. 2024. PMID: 39185425 Free PMC article. Review.
-
Tackling Immune Targets for Breast Cancer: Beyond PD-1/PD-L1 Axis.Front Oncol. 2021 Mar 5;11:628138. doi: 10.3389/fonc.2021.628138. eCollection 2021. Front Oncol. 2021. PMID: 33747948 Free PMC article. Review.
-
Nano-Strategies to Target Breast Cancer-Associated Fibroblasts: Rearranging the Tumor Microenvironment to Achieve Antitumor Efficacy.Int J Mol Sci. 2019 Mar 13;20(6):1263. doi: 10.3390/ijms20061263. Int J Mol Sci. 2019. PMID: 30871158 Free PMC article. Review.
-
GM-CSF augmented the photothermal immunotherapeutic outcome of self-driving gold nanoparticles against a mouse CT-26 colon tumor model.Biomater Res. 2023 Oct 23;27(1):105. doi: 10.1186/s40824-023-00430-6. Biomater Res. 2023. PMID: 37872620 Free PMC article.
-
Simultaneous Reversal of T Lymphocytes and Cancer Cells Metabolism Via a Biomimetic Heavy-Atom-Free Photosensitizers-Based Combination Therapies to Boost Cancer Photoimmunotherapy.Adv Sci (Weinh). 2025 Apr;12(16):e2416143. doi: 10.1002/advs.202416143. Epub 2025 Mar 5. Adv Sci (Weinh). 2025. PMID: 40042072 Free PMC article.
References
-
- Edwards B. K.; Ward E.; Kohler B. A.; Eheman C.; Zauber A. G.; Anderson R. N.; Jemal A.; Schymura M. J.; Lansdorp-Vogelaar I.; Seeff L. C.; van Ballegooijen M. C.; Goede S. L.; Ries L. A. G. Annual Report to the Nation on the Status of Cancer, 1975–2006, Featuring Colorectal Cancer Trends and Impact of Interventions (risk factors, screening, and treatment) to Reduce Future Rates. Cancer 2010, 116, 544–573. 10.1002/cncr.24760. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

