Functional optical coherence tomography of retinal photoreceptors
- PMID: 30482040
- PMCID: PMC6348599
- DOI: 10.1177/1535370218816517
Functional optical coherence tomography of retinal photoreceptors
Abstract
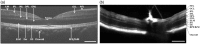
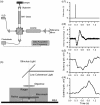
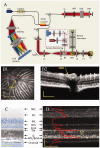
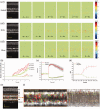
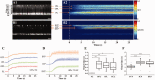
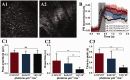
Retinal photoreceptors are the primary target of age-related macular degeneration (AMD) which is the leading cause of severe vision loss and legal blindness. An objective method for functional assessment of photoreceptor physiology can benefit early detection and better treatment evaluation of AMD and other eye diseases that are known to cause photoreceptor dysfunctions. This article summarizes in vitro study of IOS mechanisms and in vivo demonstration of IOS imaging of intact animals. Further development of the functional IOS imaging may provide a revolutionary solution to achieve objective assessment of human photoreceptors.
Keywords: Optical coherence tomography; age-related macular degeneration; intrinsic optical signal; photoreceptors.
Figures




Similar articles
-
Fast intrinsic optical signal correlates with activation phase of phototransduction in retinal photoreceptors.Exp Biol Med (Maywood). 2020 Jul;245(13):1087-1095. doi: 10.1177/1535370220935406. Epub 2020 Jun 19. Exp Biol Med (Maywood). 2020. PMID: 32558598 Free PMC article. Review.
-
Mitochondrial oxidative stress in the retinal pigment epithelium (RPE) led to metabolic dysfunction in both the RPE and retinal photoreceptors.Redox Biol. 2019 Jun;24:101201. doi: 10.1016/j.redox.2019.101201. Epub 2019 Apr 20. Redox Biol. 2019. PMID: 31039480 Free PMC article.
-
Retinal Layer Thicknesses in Early Age-Related Macular Degeneration: Results From the German AugUR Study.Invest Ophthalmol Vis Sci. 2019 Apr 1;60(5):1581-1594. doi: 10.1167/iovs.18-25332. Invest Ophthalmol Vis Sci. 2019. PMID: 30995315 Free PMC article.
-
Functional optical coherence tomography enables in vivo physiological assessment of retinal rod and cone photoreceptors.Sci Rep. 2015 Apr 22;5:9595. doi: 10.1038/srep09595. Sci Rep. 2015. PMID: 25901915 Free PMC article.
-
OPTICAL COHERENCE TOMOGRAPHY AND HISTOLOGY OF AGE-RELATED MACULAR DEGENERATION SUPPORT MITOCHONDRIA AS REFLECTIVITY SOURCES.Retina. 2018 Mar;38(3):445-461. doi: 10.1097/IAE.0000000000001946. Retina. 2018. PMID: 29210936 Free PMC article. Review.
Cited by
-
Fast intrinsic optical signal correlates with activation phase of phototransduction in retinal photoreceptors.Exp Biol Med (Maywood). 2020 Jul;245(13):1087-1095. doi: 10.1177/1535370220935406. Epub 2020 Jun 19. Exp Biol Med (Maywood). 2020. PMID: 32558598 Free PMC article. Review.
-
Extraction of phase-based optoretinograms (ORG) from serial B-scans acquired over tens of seconds by mouse retinal raster scanning OCT system.Biomed Opt Express. 2021 Nov 30;12(12):7849-7871. doi: 10.1364/BOE.439900. eCollection 2021 Dec 1. Biomed Opt Express. 2021. PMID: 35003871 Free PMC article.
-
Super-resolution ophthalmoscopy: Virtually structured detection for resolution improvement in retinal imaging.Exp Biol Med (Maywood). 2021 Feb;246(3):249-259. doi: 10.1177/1535370220970533. Epub 2020 Nov 27. Exp Biol Med (Maywood). 2021. PMID: 33243006 Free PMC article. Review.
-
Vascular morphology and blood flow signatures for differential artery-vein analysis in optical coherence tomography of the retina.Biomed Opt Express. 2020 Dec 15;12(1):367-379. doi: 10.1364/BOE.413149. eCollection 2021 Jan 1. Biomed Opt Express. 2020. PMID: 33520388 Free PMC article.
-
Functional regulation of an outer retina hyporeflective band on optical coherence tomography images.Sci Rep. 2021 May 13;11(1):10260. doi: 10.1038/s41598-021-89599-1. Sci Rep. 2021. PMID: 33986362 Free PMC article.
References
-
- Simple Anatomy of the Retina by Helga Kolb, http://webvision.med.utah.edu/book/part-i-foundations/simple-anatomy-of-... (2011, accessed 10 October 2018)
-
- Friedman DS, O'Colmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT, Nemesure B, Mitchell P, Kempen J; Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol 2004; 122:564–72 - PubMed
-
- Ishikawa K, Nishihara H, Ozawa S, Piao CH, Ito Y, Kondo M, Terasaki H. Focal macular electroretinograms after photodynamic therapy combined with intravitreal bevacizumab. Graefes Arch Clin Exp Ophthalmol 2011; 249:273–80 - PubMed
-
- Feigl B, Morris CP. The challenge of predicting macular degeneration. Curr Med Res Opin 2011; 27:1745–8 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

