Multi-arm multi-stage trials can improve the efficiency of finding effective treatments for stroke: a case study
- PMID: 30482176
- PMCID: PMC6260683
- DOI: 10.1186/s12872-018-0956-4
Multi-arm multi-stage trials can improve the efficiency of finding effective treatments for stroke: a case study
Abstract
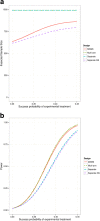
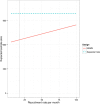
Background: Many recent Stroke trials fail to show a beneficial effect of the intervention late in the development. Currently a large number of new treatment options are being developed. Multi-arm multi-stage (MAMS) designs offer one potential strategy to avoid lengthy studies of treatments without beneficial effects while at the same time allowing evaluation of several novel treatments. In this paper we provide a review of what MAMS designs are and argue that they are of particular value for Stroke trials. We illustrate this benefit through a case study based on previous published trials of endovascular treatment for acute ischemic stroke. We show in this case study that MAMS trials provide additional power for the same sample size compared to alternative trial designs. This level of additional power depends on the recruitment length of the trial, with most efficiency gained when recruitment is relatively slow. We conclude with a discussion of additional considerations required when starting a MAMS trial.
Conclusion: MAMS trial designs are potentially very useful for stroke trials due to their improved statistical power compared to the traditional approach.
Keywords: Adaptive design; Clinical trial design; Multi-arm multi-stage trials; Multi-arm trials.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

