In vitro and in vivo accumulation of magnetic nanoporous silica nanoparticles on implant materials with different magnetic properties
- PMID: 30482189
- PMCID: PMC6258308
- DOI: 10.1186/s12951-018-0422-6
In vitro and in vivo accumulation of magnetic nanoporous silica nanoparticles on implant materials with different magnetic properties
Abstract
Background: In orthopedic surgery, implant-associated infections are still a major problem. For the improvement of the selective therapy in the infection area, magnetic nanoparticles as drug carriers are promising when used in combination with magnetizable implants and an externally applied magnetic field. These implants principally increase the strength of the magnetic field resulting in an enhanced accumulation of the drug loaded particles in the target area and therewith a reduction of the needed amount and the risk of undesirable side effects. In the present study magnetic nanoporous silica core-shell nanoparticles, modified with fluorophores (fluorescein isothiocyanate/FITC or rhodamine B isothiocyanate/RITC) and poly(ethylene glycol) (PEG), were used in combination with metallic plates of different magnetic properties and with a magnetic field. In vitro and in vivo experiments were performed to investigate particle accumulation and retention and their biocompatibility.
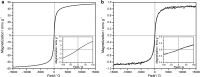
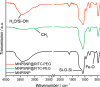
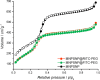
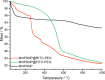
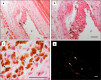
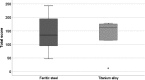
Results: Spherical magnetic silica core-shell nanoparticles with reproducible superparamagnetic behavior and high porosity were synthesized. Based on in vitro proliferation and viability tests the modification with organic fluorophores and PEG led to highly biocompatible fluorescent particles, and good dispersibility. In a circular tube system martensitic steel 1.4112 showed superior accumulation and retention of the magnetic particles in comparison to ferritic steel 1.4521 and a Ti90Al6V4 control. In vivo tests in a mouse model where the nanoparticles were injected subcutaneously showed the good biocompatibility of the magnetic silica nanoparticles and their accumulation on the surface of a metallic plate, which had been implanted before, and in the surrounding tissue.
Conclusion: With their superparamagnetic properties and their high porosity, multifunctional magnetic nanoporous silica nanoparticles are ideal candidates as drug carriers. In combination with their good biocompatibility in vitro, they have ideal properties for an implant directed magnetic drug targeting. Missing adverse clinical and histological effects proved the good biocompatibility in vivo. Accumulation and retention of the nanoparticles could be influenced by the magnetic properties of the implanted plates; a remanent martensitic steel plate significantly improved both values in vitro. Therefore, the use of magnetizable implant materials in combination with the magnetic nanoparticles has promising potential for the selective treatment of implant-associated infections.
Keywords: Biocompatibility; Core–shell nanoparticles; Drug targeting; Ferritic steel; Martensitic steel; Mouse model; Nanoporous silica; PEGylation; Superparamagnetic Fe3O4.
Figures


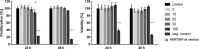
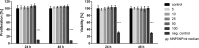



Similar articles
-
Preparation and PET/CT imaging of implant directed 68Ga-labeled magnetic nanoporous silica nanoparticles.J Nanobiotechnology. 2023 Aug 17;21(1):270. doi: 10.1186/s12951-023-02041-8. J Nanobiotechnology. 2023. PMID: 37592318 Free PMC article.
-
Biodistribution, biocompatibility and targeted accumulation of magnetic nanoporous silica nanoparticles as drug carrier in orthopedics.J Nanobiotechnology. 2020 Jan 15;18(1):14. doi: 10.1186/s12951-020-0578-8. J Nanobiotechnology. 2020. PMID: 31941495 Free PMC article.
-
Increased accumulation of magnetic nanoparticles by magnetizable implant materials for the treatment of implant-associated complications.J Nanobiotechnology. 2013 Oct 10;11:34. doi: 10.1186/1477-3155-11-34. J Nanobiotechnology. 2013. PMID: 24112871 Free PMC article.
-
SYNTHESIS AND APPLICATIONS OF Fe3O4/SiO2 CORE-SHELL MATERIALS.Curr Pharm Des. 2015;21(37):5324-35. doi: 10.2174/1381612821666150917094031. Curr Pharm Des. 2015. PMID: 26377652 Review.
-
pH sensitive core-shell magnetic nanoparticles for targeted drug delivery in cancer therapy.Rom J Morphol Embryol. 2016;57(1):23-32. Rom J Morphol Embryol. 2016. PMID: 27151685 Review.
Cited by
-
Preparation and PET/CT imaging of implant directed 68Ga-labeled magnetic nanoporous silica nanoparticles.J Nanobiotechnology. 2023 Aug 17;21(1):270. doi: 10.1186/s12951-023-02041-8. J Nanobiotechnology. 2023. PMID: 37592318 Free PMC article.
-
Comparison of accumulation and distribution of PEGylated and CD-47-functionalized magnetic nanoporous silica nanoparticles in an in vivo mouse model of implant infection.PLoS One. 2025 May 2;20(5):e0321888. doi: 10.1371/journal.pone.0321888. eCollection 2025. PLoS One. 2025. PMID: 40315195 Free PMC article.
-
Biodistribution, biocompatibility and targeted accumulation of magnetic nanoporous silica nanoparticles as drug carrier in orthopedics.J Nanobiotechnology. 2020 Jan 15;18(1):14. doi: 10.1186/s12951-020-0578-8. J Nanobiotechnology. 2020. PMID: 31941495 Free PMC article.
-
Understanding the mechanisms of silica nanoparticles for nanomedicine.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2021 Jan;13(1):e1658. doi: 10.1002/wnan.1658. Epub 2020 Jun 29. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2021. PMID: 32602269 Free PMC article. Review.
-
Influence of PEG Chain Length of Functionalized Magnetic Nanoparticles on the Cytocompatibility and Immune Competence of Primary Murine Macrophages and Dendritic Cells.Int J Mol Sci. 2023 Jan 29;24(3):2565. doi: 10.3390/ijms24032565. Int J Mol Sci. 2023. PMID: 36768890 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

