Universal CARs, universal T cells, and universal CAR T cells
- PMID: 30482221
- PMCID: PMC6257951
- DOI: 10.1186/s13045-018-0677-2
Universal CARs, universal T cells, and universal CAR T cells
Abstract
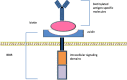
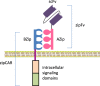
Currently, the two approved T cell products with chimeric antigen receptors (CAR) are from autologous T cells. These CAR T cells approved for clinical use must be generated on a custom-made basis. This case-by-case autologous T cell production platform remains a significant limiting factor for large-scale clinical application due to the costly and lengthy production process. There is also an inherent risk of production failure. The individualized, custom-made autologous CAR T cell production process also posts constriction on the wide application on diverse tumor types. Therefore, universal allogeneic T cells are needed for the preparation of universal CAR T cells that can serve as the "off-the-shelf" ready-to-use therapeutic agents for large-scale clinical applications. Genome-editing technologies including ZFN (zinc finger nuclease), TALEN (transcription activator-like effector nuclease), and CRISPR-Cas9 are being used to generate the universal third-party T cells. In addition, split, universal, and programmable (SUPRA) CARs are being developed to enhance the flexibility and controllability of CAR T cells. The engineered universal T cells and universal CARs are paving the road for a totally new generation of CAR T cells capable of targeting multiple antigens and/ or being delivered to multiple recipients without re-editing of T cells. This may escalate to a new wave of revolution in cancer immunotherapy. This review summarized the latest advances on designs and development of universal CARs, universal T cells, and clinical application of universal CAR T cells.
Conflict of interest statement
Ethics approval and consent to participate
This is not applicable for this review.
Consent for publication
This is not applicable for this review.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




Similar articles
-
Use of Cell and Genome Modification Technologies to Generate Improved "Off-the-Shelf" CAR T and CAR NK Cells.Front Immunol. 2020 Aug 7;11:1965. doi: 10.3389/fimmu.2020.01965. eCollection 2020. Front Immunol. 2020. PMID: 32903482 Free PMC article. Review.
-
Significant Advancements and Evolutions in Chimeric Antigen Receptor Design.Int J Mol Sci. 2024 Nov 13;25(22):12201. doi: 10.3390/ijms252212201. Int J Mol Sci. 2024. PMID: 39596267 Free PMC article. Review.
-
CRISPR/Cas9 and CAR-T cell, collaboration of two revolutionary technologies in cancer immunotherapy, an instruction for successful cancer treatment.Hum Immunol. 2018 Dec;79(12):876-882. doi: 10.1016/j.humimm.2018.09.007. Epub 2018 Sep 24. Hum Immunol. 2018. PMID: 30261221 Review.
-
Off-the-Shelf Allogeneic T Cell Therapies for Cancer: Opportunities and Challenges Using Naturally Occurring "Universal" Donor T Cells.Front Immunol. 2020 Nov 11;11:583716. doi: 10.3389/fimmu.2020.583716. eCollection 2020. Front Immunol. 2020. PMID: 33262761 Free PMC article. Review.
-
The Evolving Protein Engineering in the Design of Chimeric Antigen Receptor T Cells.Int J Mol Sci. 2019 Dec 27;21(1):204. doi: 10.3390/ijms21010204. Int J Mol Sci. 2019. PMID: 31892219 Free PMC article. Review.
Cited by
-
Programmable and multi-targeted CARs: a new breakthrough in cancer CAR-T cell therapy.Clin Transl Oncol. 2021 Jun;23(6):1003-1019. doi: 10.1007/s12094-020-02490-9. Epub 2020 Sep 30. Clin Transl Oncol. 2021. PMID: 32997278 Review.
-
Strategies to enhance CAR-T persistence.Biomark Res. 2022 Nov 23;10(1):86. doi: 10.1186/s40364-022-00434-9. Biomark Res. 2022. PMID: 36419115 Free PMC article. Review.
-
In-Vivo Induced CAR-T Cell for the Potential Breakthrough to Overcome the Barriers of Current CAR-T Cell Therapy.Front Oncol. 2022 Feb 10;12:809754. doi: 10.3389/fonc.2022.809754. eCollection 2022. Front Oncol. 2022. PMID: 35223491 Free PMC article. Review.
-
[Relapse mechanism and coping strategies of CD19 chimeric antigen receptor T cells in the treatment of diffuse large B-cell lymphoma].Zhonghua Xue Ye Xue Za Zhi. 2022 Sep 14;43(9):788-792. doi: 10.3760/cma.j.issn.0253-2727.2022.09.014. Zhonghua Xue Ye Xue Za Zhi. 2022. PMID: 36709176 Free PMC article. Chinese. No abstract available.
-
Optimizing sgRNA to Improve CRISPR/Cas9 Knockout Efficiency: Special Focus on Human and Animal Cell.Front Bioeng Biotechnol. 2021 Nov 19;9:775309. doi: 10.3389/fbioe.2021.775309. eCollection 2021. Front Bioeng Biotechnol. 2021. Retraction in: Front Bioeng Biotechnol. 2023 Sep 04;11:1285036. doi: 10.3389/fbioe.2023.1285036. PMID: 34869290 Free PMC article. Retracted. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

