Abnormally elevated USP37 expression in breast cancer stem cells regulates stemness, epithelial-mesenchymal transition and cisplatin sensitivity
- PMID: 30482232
- PMCID: PMC6258492
- DOI: 10.1186/s13046-018-0934-9
Abnormally elevated USP37 expression in breast cancer stem cells regulates stemness, epithelial-mesenchymal transition and cisplatin sensitivity
Erratum in
-
Correction to: Abnormally elevated USP37 expression in breast cancer stem cells regulates stemness, epithelial-mesenchymal transition and cisplatin sensitivity.J Exp Clin Cancer Res. 2021 Nov 9;40(1):354. doi: 10.1186/s13046-021-02158-4. J Exp Clin Cancer Res. 2021. PMID: 34753507 Free PMC article. No abstract available.
Abstract
Background: Recent studies have indicated that deubiquitinating enzymes (DUBs) are related to the stem-cell pathway network and chemo-resistance in cancer. Ubiquitin-specific peptidase 37 (USP37), a novel DUB, was identified to be a potential factor associated with tumor progression. However, the biological functions of USP37 in breast cancer remain unclear.
Methods: The distribution of USP37 expression in breast cancer and the correlation between USP37 expression and the overall survival rate were detected by The Cancer Genome Atlas (TCGA) database. Gene set enrichment analysis (GSEA) was utilized to evaluate potential mechanism of USP37 in breast cancer. The USP37 expression in breast cancer tissues and breast cancer cell lines were detected by immunohistochemistry and western blotting. Sorting of breast cancer stem cells (BCSCs) were by using MACS assay. In vitro and in vivo assays were performed to examine the biological functions of USP37 in breast cancer cells. MG132, CHX chase, immunofluorescence staining and co-immunoprecipitation assays were used to test the interaction between USP37 and Gli-1.
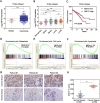
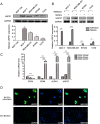
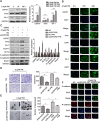
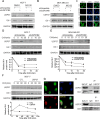
Results: Bioinformatics analysis demonstrated that USP37 gene was elevated in breast cancer tissues and its overexpression was strongly correlated with the increased mortality rate. GSEA analysis showed that USP37 expression was positively associated with cell growth and metastasis while negatively related to cell apoptosis in the TCGA breast cancer samples. USP37 expression was elevated in breast cancer tissues and breast cancer cell lines. Moreover, we also detected that USP37 was overexpressed in BCSCs. USP37 regulated the ability of cell invasion, epithelial-mesenchymal transition (EMT), stemness and cisplatin sensitivity in breast cancer cell lines. Additionally, USP37 knockdown inhibited tumorigenicity and increased anticancer effect of cisplatin in vivo. Knockdown of USP37 significantly decreased hedgehog (Hh) pathway components Smo and Gli-1. Gli-1 was stabilized by USP37 and they interacted with each other. Further studies indicated that USP37 knockdown could inhibit the stemness, cell invasion and EMT in breast cancer via downregulation of Hh pathway.
Conclusions: These findings reveal that USP37 is highly expressed in BCSCs and is correlated with poor prognosis in breast cancer patients. USP37 can regulate the stemness, cell invasion and EMT via Hh pathway, and decreased USP37 confers sensitivity to cisplatin in breast cancer cells. USP37 is required for the regulation of breast cancer progression, as well as a critical target for clinical treatment of breast cancer.
Keywords: Breast cancer; Cisplatin; EMT; Hedgehog; Stemness; USP37.
Conflict of interest statement
Ethics approval and consent to participate
This research was reviewed and approved by the Ethical Committee and Institutional Review Board of Dalian Medical University. Animal experiments and procedures were approved by the Animal Care and Use Committee of Dalian Medical University and performed in accordance with the Guide for the Care and Use of Laboratory Animals (NIH).(No. AEE17046). The tissue samples of patients diagnosed with breast cancer were used in this research provided written informed consent, and the study protocol was approved by the Committee for the Ethical Review of Research, The Second Affiliated Hospital of Dalian Medical University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



Similar articles
-
USP37 downregulation elevates the Chemical Sensitivity of Human Breast Cancer Cells to Adriamycin.Int J Med Sci. 2021 Jan 1;18(2):325-334. doi: 10.7150/ijms.54301. eCollection 2021. Int J Med Sci. 2021. PMID: 33390801 Free PMC article.
-
Bmi1 regulates self-renewal and epithelial to mesenchymal transition in breast cancer cells through Nanog.BMC Cancer. 2014 Oct 28;14:785. doi: 10.1186/1471-2407-14-785. BMC Cancer. 2014. PMID: 25348805 Free PMC article.
-
LincK contributes to breast tumorigenesis by promoting proliferation and epithelial-to-mesenchymal transition.J Hematol Oncol. 2019 Feb 22;12(1):19. doi: 10.1186/s13045-019-0707-8. J Hematol Oncol. 2019. PMID: 30795783 Free PMC article.
-
Ubiquitin-specific peptidase 37: an important cog in the oncogenic machinery of cancerous cells.J Exp Clin Cancer Res. 2021 Nov 10;40(1):356. doi: 10.1186/s13046-021-02163-7. J Exp Clin Cancer Res. 2021. PMID: 34758854 Free PMC article. Review.
-
Metabolic reprogramming on breast cancer stem Cells: Proliferation and self-renewal, epithelial-mesenchymal transition (EMT), and drug resistance.Biochem Biophys Res Commun. 2025 Aug 8;774:152079. doi: 10.1016/j.bbrc.2025.152079. Epub 2025 May 23. Biochem Biophys Res Commun. 2025. PMID: 40446745 Review.
Cited by
-
Stabilization of estrogen receptor α by USP37 contributes to the progression of breast cancer.Cancer Sci. 2023 May;114(5):2041-2052. doi: 10.1111/cas.15613. Epub 2023 Mar 10. Cancer Sci. 2023. PMID: 36221793 Free PMC article.
-
Emerging potential of ubiquitin-specific proteases and ubiquitin-specific proteases inhibitors in breast cancer treatment.World J Clin Cases. 2022 Nov 16;10(32):11690-11701. doi: 10.12998/wjcc.v10.i32.11690. World J Clin Cases. 2022. PMID: 36405275 Free PMC article. Review.
-
Correlation of breast cancer microcirculation construction with tumor stem cells (CSCs) and epithelial-mesenchymal transition (EMT) based on contrast-enhanced ultrasound (CEUS).PLoS One. 2021 Dec 21;16(12):e0261138. doi: 10.1371/journal.pone.0261138. eCollection 2021. PLoS One. 2021. PMID: 34932597 Free PMC article.
-
DUBs Activating the Hedgehog Signaling Pathway: A Promising Therapeutic Target in Cancer.Cancers (Basel). 2020 Jun 10;12(6):1518. doi: 10.3390/cancers12061518. Cancers (Basel). 2020. PMID: 32531973 Free PMC article. Review.
-
miR-4487 Enhances Gefitinib-Mediated Ubiquitination and Autophagic Degradation of EGFR in Non-Small Cell Lung Cancer Cells by Targeting USP37.Cancer Res Treat. 2022 Apr;54(2):445-457. doi: 10.4143/crt.2021.622. Epub 2021 Aug 3. Cancer Res Treat. 2022. PMID: 34352998 Free PMC article.
References
-
- Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, Rimm DL, Wong H, Rodriguez A, Herschkowitz JI, Fan C, Zhang X, He X, Pavlick A, Gutierrez MC, Renshaw L, Larionov AA, Faratian D, Hilsenbeck SG, Perou CM, Lewis MT, Rosen JM, Chang JC. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A. 2009;106(33):13820–13825. doi: 10.1073/pnas.0905718106. - DOI - PMC - PubMed
-
- Ricardo S, Vieira AF, Gerhard R, Leitão D, Pinto R, Cameselle-Teijeiro JF, Milanezi F, Schmitt F, Paredes J. Breast cancer stem cell markers CD44, CD24 and ALDH1: expression distribution within intrinsic molecular subtype. J Clin Pathol. 2011;64(11):937–946. doi: 10.1136/jcp.2011.090456. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

