Development of a Core Outcome Set for Studies on Obesity in Pregnant Patients (COSSOPP): a study protocol
- PMID: 30482249
- PMCID: PMC6258169
- DOI: 10.1186/s13063-018-3029-1
Development of a Core Outcome Set for Studies on Obesity in Pregnant Patients (COSSOPP): a study protocol
Abstract
Background: Maternal obesity is a risk factor for adverse maternal, fetal, and neonatal events. Numerous clinical trials are currently exploring the effectiveness of antenatal and peripartum interventions in improving pregnancy outcomes that can in future inform clinical practice. However, the heterogeneity in outcome reporting limits our ability to compare outcomes across studies, and there is a lack of stakeholder representation in outcome choice. A pragmatic solution to this problem is the development of a core outcome set (COS) that defines the minimum criteria for outcome reporting in clinical trials undertaken in this population, arrived at by the involvement of relevant stakeholders.
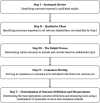
Methods: The development of a COS for studies on obesity in pregnant patients (COSSOPP) will comprise five steps. First, a systematic review of published literature will identify the long list of outcomes, their definitions and measurements if applicable, and outcome reporting quality. This will be followed by a meta-synthesis of qualitative studies with patients, and qualitative interviews in Toronto with patients, clinicians, researchers, hospital administrators, and policy-makers, to identify novel outcomes that were not obtained through systematic review. Third, the long list of outcomes will be narrowed down through online Delphi surveys involving an international group of patients and relevant stakeholders. This will be followed by a face-to-face consensus meeting with representatives of all stakeholder groups to arrive at a consensus on the final COS. Finally, in order to determine how the identified core outcomes should be measured, we will conduct another literature review and Delphi process.
Discussion: COSSOPP will engage patients, clinicians, researchers, and other relevant stakeholders in determining the core set of outcomes that should be reported and measured in order to harmonize outcome reporting in studies evaluating the effectiveness of antepartum and peripartum interventions in obese pregnant women. This protocol provides a detailed overview of the steps involved in the development of a COS, to guide researchers in developing COS within their areas of specialization. COMET CORE OUTCOME SET REGISTRATION: http://www.comet-initiative.org/studies/details/939 .
Keywords: Childbirth; Consensus; Core outcome set; Delphi; Obesity; Outcome reporting; Patient-reported outcomes; Pregnancy; Qualitative research; Stakeholder.
Conflict of interest statement
Ethics approval and consent to participate
This study and the consent forms for stakeholder participation have received approval from the Mount Sinai Hospital Research Ethics Board (#17–0316-E). Consent will be obtained in writing for in-person methods by author RDa (focus groups, face-to-face individual interviews and the consensus meeting), electronically for the Delphi method, and verbally for individual telephone interviews.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Ogden CL, Carroll M, Kit B, Flegal K. Prevalence of obesity in the United States, 2009–2010. NCHS data brief, no. 82. Hyattsville: National Center for Health Statistics; 2012. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical