Acute flaccid paralysis (AFP) surveillance: challenges and opportunities from 18 years' experience, Spain, 1998 to 2015
- PMID: 30482263
- PMCID: PMC6341937
- DOI: 10.2807/1560-7917.ES.2018.23.47.1700423
Acute flaccid paralysis (AFP) surveillance: challenges and opportunities from 18 years' experience, Spain, 1998 to 2015
Abstract
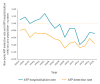
Acute flaccid paralysis (AFP) surveillance is key for global polio eradication. It allows detecting poliovirus (PV) reintroductions from endemic countries. This study describes AFP surveillance in Spain from 1998 to 2015. During this time, 678 AFP cases were reported to the Spanish National Surveillance Network. The mean notification rate was 0.58 AFP cases/100,000 population under 15 years old (range: 0.45/100,000-0.78/100,000). Two periods (P) are described: P1 (1998-2006) with the AFP notification rate ranging from 0.66/100,000 to 0.78/100,000, peaking in 2001 (0.84/100,000); and P2 (2007-2015) when the AFP rate ranged from 0.43/100,000 to 0.57/100,000, with the lowest rate in 2009 (0.31/100,000). No poliomyelitis cases were caused by wild PV infections, although two Sabin-like PVs and one imported vaccine-derived PV-2 were detected. Overall, 23 (3.4%) cases met the hot case definition. Most cases were clinically diagnosed with Guillain-Barré syndrome (76.9%; 504/655). The adequate stool collection rate ranged from 33.3% (7/21) to 72.5% (29/40). The annual proportion of AFP cases with non-polio enterovirus findings varied widely across the study period. AFP surveillance with laboratory testing for non-polio enteroviruses must be maintained and enhanced both to monitor polio eradication and to establish sensitive surveillance for prompt detection of other enteroviruses causing serious symptoms.
Keywords: Spain; acute flaccid paralysis surveillance; enterovirus; poliomyelitis eradication; surveillance; vaccine preventable disease.
Conflict of interest statement
Figures



References
-
- World Health Organization (WHO). Acute flaccid paralysis (AFP) surveillance: the surveillance strategy for poliomyelitis eradication. Weekly Epidemiological Record. Geneva: WHO; 1998. Available from: http://www.who.int/docstore/wer/pdf/1998/wer7316.pdf
-
- World Health Organization (WHO). WHO statement on the 8th IHR Emergency Committee meeting regarding the international spread of poliovirus. Geneva: WHO; 2016. [Accessed 10 Dec 2016]. Available from: http://www.who.int/mediacentre/news/statements/2016/8th-IHR-emergency-co...
-
- World Health Organization (WHO). WHO statement on the meeting of the International Health Regulations Emergency Committee concerning the International spread of wild poliovirus. Geneva: WHO; 2014. [Accessed 20 Dec 2016]. Available from: http://www.who.int/mediacentre/news/statements/2014/polio-20140505/en/
-
- Centers for Disease Control and Prevention (CDC) Update on vaccine-derived polioviruses--worldwide, January 2008-June 2009. MMWR Morb Mortal Wkly Rep. 2009;58(36):1002-6. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
