Posing the APC/C E3 Ubiquitin Ligase to Orchestrate Cell Division
- PMID: 30482618
- PMCID: PMC6340778
- DOI: 10.1016/j.tcb.2018.09.007
Posing the APC/C E3 Ubiquitin Ligase to Orchestrate Cell Division
Abstract
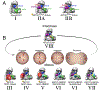
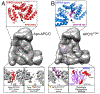
The anaphase promoting complex/cyclosome (APC/C) E3 ligase controls mitosis and nonmitotic pathways through interactions with proteins that coordinate ubiquitylation. Since the discovery that the catalytic subunits of APC/C are conformationally dynamic cullin and RING proteins, many unexpected and intricate regulatory mechanisms have emerged. Here, we review structural knowledge of this regulation, focusing on: (i) coactivators, E2 ubiquitin (Ub)-conjugating enzymes, and inhibitors engage or influence multiple sites on APC/C including the cullin-RING catalytic core; and (ii) the outcomes of these interactions rely on mobility of coactivators and cullin-RING domains, which permits distinct conformations specifying different functions. Thus, APC/C is not simply an interaction hub, but is instead a dynamic, multifunctional molecular machine whose structure is remodeled by binding partners to achieve temporal ubiquitylation regulating cell division.
Keywords: E3 ligase; anaphase promoting complex/cyclosome; cell division; cryo-electron microscopy; mitosis; ubiquitin.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures
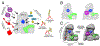

References
-
- King RW et al. (1996) How proteolysis drives the cell cycle. Science 274 (5293), 1652–9. - PubMed
-
- King RW et al. (1995) A 20S complex containing CDC27 and CDC16 catalyzes the mitosis-specific conjugation of ubiquitin to cyclin B. Cell 81 (2), 279–88. - PubMed
-
- Irniger S et al. (1995) Genes involved in sister chromatid separation are needed for B-type cyclin proteolysis in budding yeast. Cell 81 (2), 269–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

