A Self-Regulating Gap Junction Network of Amacrine Cells Controls Nitric Oxide Release in the Retina
- PMID: 30482690
- PMCID: PMC6317889
- DOI: 10.1016/j.neuron.2018.09.047
A Self-Regulating Gap Junction Network of Amacrine Cells Controls Nitric Oxide Release in the Retina
Abstract
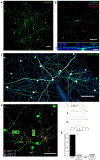
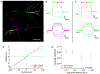
Neuromodulators regulate circuits throughout the nervous system, and revealing the cell types and stimulus conditions controlling their release is vital to understanding their function. The effects of the neuromodulator nitric oxide (NO) have been studied in many circuits, including in the vertebrate retina, where it regulates synaptic release, gap junction coupling, and blood vessel dilation, but little is known about the cells that release NO. We show that a single type of amacrine cell (AC) controls NO release in the inner retina, and we report its light responses, electrical properties, and calcium dynamics. We discover that this AC forms a dense gap junction network and that the strength of electrical coupling in the network is regulated by light through NO. A model of the network offers insights into the biophysical specializations leading to auto-regulation of NO release within the network.
Keywords: Connexin-45; NOS; amacrine cell; gap junctions; light adaptation; nNOS; nNOS-2; nitric oxide; retina.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures

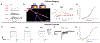


Similar articles
-
Amacrine Cells Forming Gap Junctions With Intrinsically Photosensitive Retinal Ganglion Cells: ipRGC Types, Neuromodulator Contents, and Connexin Isoform.Invest Ophthalmol Vis Sci. 2021 Jan 4;62(1):10. doi: 10.1167/iovs.62.1.10. Invest Ophthalmol Vis Sci. 2021. PMID: 33410914 Free PMC article.
-
Dopamine-stimulated dephosphorylation of connexin 36 mediates AII amacrine cell uncoupling.J Neurosci. 2009 Nov 25;29(47):14903-11. doi: 10.1523/JNEUROSCI.3436-09.2009. J Neurosci. 2009. PMID: 19940186 Free PMC article.
-
Light adaptation in the chick retina: Dopamine, nitric oxide, and gap-junction coupling modulate spatiotemporal contrast sensitivity.Exp Eye Res. 2020 Jun;195:108026. doi: 10.1016/j.exer.2020.108026. Epub 2020 Apr 1. Exp Eye Res. 2020. PMID: 32246982
-
Multiple neuronal connexins in the mammalian retina.Cell Commun Adhes. 2003 Jul-Dec;10(4-6):425-30. doi: 10.1080/cac.10.4-6.425.430. Cell Commun Adhes. 2003. PMID: 14681052 Review.
-
Neuronal nitric oxide synthase immunoreactive neurons in the mammalian retina.Microsc Res Tech. 2000 Jul 15;50(2):112-23. doi: 10.1002/1097-0029(20000715)50:2<112::AID-JEMT3>3.0.CO;2-S. Microsc Res Tech. 2000. PMID: 10891875 Review.
Cited by
-
Hemodynamic Response of the Three Macular Capillary Plexuses in Dark Adaptation and Flicker Stimulation Using Optical Coherence Tomography Angiography.Invest Ophthalmol Vis Sci. 2019 Feb 1;60(2):694-703. doi: 10.1167/iovs.18-25478. Invest Ophthalmol Vis Sci. 2019. PMID: 30786274 Free PMC article.
-
Acute Hyperglycemia Reverses Neurovascular Coupling During Dark to Light Adaptation in Healthy Subjects on Optical Coherence Tomography Angiography.Invest Ophthalmol Vis Sci. 2020 Apr 9;61(4):38. doi: 10.1167/iovs.61.4.38. Invest Ophthalmol Vis Sci. 2020. PMID: 32340033 Free PMC article.
-
An offset ON-OFF receptive field is created by gap junctions between distinct types of retinal ganglion cells.Nat Neurosci. 2021 Jan;24(1):105-115. doi: 10.1038/s41593-020-00747-8. Epub 2020 Nov 23. Nat Neurosci. 2021. PMID: 33230322 Free PMC article.
-
BH4 supplementation reduces retinal cell death in ischaemic retinopathy.Sci Rep. 2023 Dec 2;13(1):21292. doi: 10.1038/s41598-023-48167-5. Sci Rep. 2023. PMID: 38042898 Free PMC article.
-
Amacrine Cells Forming Gap Junctions With Intrinsically Photosensitive Retinal Ganglion Cells: ipRGC Types, Neuromodulator Contents, and Connexin Isoform.Invest Ophthalmol Vis Sci. 2021 Jan 4;62(1):10. doi: 10.1167/iovs.62.1.10. Invest Ophthalmol Vis Sci. 2021. PMID: 33410914 Free PMC article.
References
-
- Badea TC, and Nathans J (2004). Quantitative analysis of neuronal morphologies in the mouse retina visualized by using a genetically directed reporter. J. Comp. Neurol 480, 331–351. - PubMed
-
- Benda J, Bock R, Rujan P, and Ammermüller J (2001). Asymmetrical dynamics of voltage spread in retinal horizontal cell networks. Vis. Neurosci 18, 835–848. - PubMed
-
- Berson DM, Dunn FA, and Takao M (2002). Phototransduction by retinal ganglion cells that set the circadian clock. Science 295, 1070–1073. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

