A Web-Based Telemanagement System for Improving Disease Activity and Quality of Life in Patients With Complex Inflammatory Bowel Disease: Pilot Randomized Controlled Trial
- PMID: 30482739
- PMCID: PMC6301812
- DOI: 10.2196/11602
A Web-Based Telemanagement System for Improving Disease Activity and Quality of Life in Patients With Complex Inflammatory Bowel Disease: Pilot Randomized Controlled Trial
Abstract
Background: The reported efficacy of telemedicine in patients with inflammatory bowel disease (IBD) is inconsistent among studies, and data for complex IBD are lacking.
Objective: We aimed to evaluate the impact of remote monitoring using a Web system-Telemonitorización de la Enfermedad de Crohn y Colitis Ulcerosa or Telemonitoring of Crohn's Disease and Ulcerative Colitis (TECCU)-as compared to standard care and telephone care on health outcomes and health care in patients with complex IBD.
Methods: We performed a 3-arm randomized controlled trial. Adult patients with IBD who received immunosuppressants and biological agents were recruited from the IBD Unit of a tertiary university hospital. The patients were randomized into groups to receive remote monitoring (G_TECCU), nurse-assisted telephone care (G_NT), or standard care with in-person visits (G_control). All patients completed the study visits at baseline and at 12 and 24 weeks in addition to each type of intervention. The primary outcome was the percentage of patients in remission at 24 weeks. Secondary health outcomes were quality of life, medication adherence, adverse effects, satisfaction, and social activities. Data on the number of outpatient visits and telephone calls, emergency visits, hospitalizations, IBD-related surgeries, and corticosteroid courses were also collected.
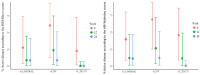
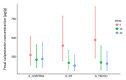
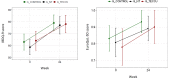
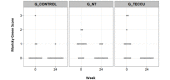
Results: A total of 63 patients were selected (21 patients in each group). During the study, 90.5% (19/21) of patients in G_control, 95.2% (20/21) in G_NT, and 85.7% (18/21) in G_TECCU were compliant to the intervention. After 24 weeks, the percentage of patients in remission was higher in G_TECCU (17/21, 81%) than in G_NT (14/21, 66.7%) and G_control (15/21, 71.4%). A higher improvement in disease activity was observed in G_TECCU than in G_control in terms of the Harvey-Bradshaw/Mayo (odds ratio=0.12, 95% CI=0.003-2.162, P=.19) and Harvey-Bradshaw/Walmsley (odds ratio=0.11, 95% CI=0.004-1.55, P=.13) indexes. Improvement in disease activity was associated with a larger reduction in fecal calprotectin values in G_TECCU compared to G_control (estimated intervention effect: odds ratio=-0.90; 95% CI=-1.96 to 0.16, P=.11). All completers adhered to treatment in G_TECCU. In addition, the quality of life, social activities, and satisfaction improved in all 3 groups. Although the number of outpatient visits and telephone calls was lower in G_TECCU than in G_NT and G_control, the safety profile was similar in all 3 groups.
Conclusions: This pilot clinical trial suggests that the TECCU Web-based system is a safe strategy for improving health outcomes in patients with complex IBD and reducing the use of health care resources.
Trial registration: ClinicalTrials.gov NCT02943538; https://clinicaltrials.gov/ct2/show/NCT02943538 (Archived by WebCite at http://www.webcitation.org/746CRRtDN).
Keywords: Crohn disease; e-health; inflammatory bowel disease; information and communication technology; telemedicine; ulcerative colitis.
©Javier Del Hoyo, Pilar Nos, Raquel Faubel, Diana Muñoz, David Domínguez, Guillermo Bastida, Bernardo Valdivieso, Marisa Correcher, Mariam Aguas. Originally published in the Journal of Medical Internet Research (http://www.jmir.org), 27.11.2018.
Conflict of interest statement
Conflicts of Interest: DD is the general manager of Connected Health Services.
Figures

References
-
- Kappelman MD, Porter CQ, Galanko JA, Rifas-Shiman SL, Ollendorf DA, Sandler RS, Finkelstein JA. Utilization of healthcare resources by U.S. children and adults with inflammatory bowel disease. Inflamm Bowel Dis. 2011 Jan;17(1):62–8. doi: 10.1002/ibd.21371. http://europepmc.org/abstract/MED/20564532 - DOI - PMC - PubMed
-
- van Deen WK, van Oijen MGH, Myers KD, Centeno A, Howard W, Choi JM, Roth BE, McLaughlin EM, Hollander D, Wong-Swanson B, Sack J, Ong MK, Ha CY, Esrailian E, Hommes DW. A nationwide 2010-2012 analysis of U.S. health care utilization in inflammatory bowel diseases. Inflamm Bowel Dis. 2014 Oct;20(10):1747–53. doi: 10.1097/MIB.0000000000000139. - DOI - PubMed
-
- Hoivik ML, Moum B, Solberg IC, Cvancarova M, Hoie O, Vatn MH, Bernklev T, IBSEN Study Group Health-related quality of life in patients with ulcerative colitis after a 10-year disease course: results from the IBSEN study. Inflamm Bowel Dis. 2012 Aug;18(8):1540–9. doi: 10.1002/ibd.21863. - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

