American Society of Hematology 2018 guidelines for management of venous thromboembolism: diagnosis of venous thromboembolism
- PMID: 30482764
- PMCID: PMC6258916
- DOI: 10.1182/bloodadvances.2018024828
American Society of Hematology 2018 guidelines for management of venous thromboembolism: diagnosis of venous thromboembolism
Abstract
Background: Modern diagnostic strategies for venous thromboembolism (VTE) incorporate pretest probability (PTP; prevalence) assessment. The ability of diagnostic tests to correctly identify or exclude VTE is influenced by VTE prevalence and test accuracy characteristics.
Objective: These evidence-based guidelines are intended to support patients, clinicians, and health care professionals in VTE diagnosis. Diagnostic strategies were evaluated for pulmonary embolism (PE), deep vein thrombosis (DVT) of the lower and upper extremity, and recurrent VTE.
Methods: The American Society of Hematology (ASH) formed a multidisciplinary panel including patient representatives. The McMaster University GRADE Centre completed systematic reviews up to 1 October 2017. The panel prioritized questions and outcomes and used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to assess evidence and make recommendations. Test accuracy estimates and VTE population prevalence were used to model expected outcomes in diagnostic pathways. Where modeling was not feasible, management and accuracy studies were used to formulate recommendations.
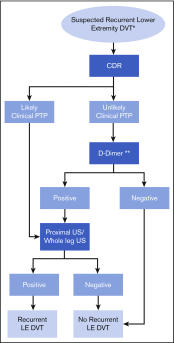
Results: Ten recommendations are presented, by PTP for patients with suspected PE and lower extremity DVT, and for recurrent VTE and upper extremity DVT.
Conclusions: For patients at low (unlikely) VTE risk, using D-dimer as the initial test reduces the need for diagnostic imaging. For patients at high (likely) VTE risk, imaging is warranted. For PE diagnosis, ventilation-perfusion scanning and computed tomography pulmonary angiography are the most validated tests, whereas lower or upper extremity DVT diagnosis uses ultrasonography. Research is needed on new diagnostic modalities and to validate clinical decision rules for patients with suspected recurrent VTE.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: All authors were members of the guideline panel or members of the systematic review team or both. As such, they completed a disclosure of interest form, which was reviewed by ASH and is available as supplements 2 and 3.
Figures





References
-
- Institute of Medicine (US) Committee on Standards for Developing Trustworthy Clinical Practice Guidelines. Clinical Practice Guidelines We Can Trust. Washington, DC: National Academies Press; 2011.
-
- Qaseem A, Forland F, Macbeth F, Ollenschläger G, Phillips S, van der Wees P; Board of Trustees of the Guidelines International Network. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156(7):525-531. - PubMed
-
- Schünemann HJ, Al-Ansary LA, Forland F, et al. ; Board of Trustees of the Guidelines International Network. Guidelines International Network: principles for disclosure of interests and management of conflicts in guidelines. Ann Intern Med. 2015;163(7):548-553. - PubMed
-
- Langer G, Meerpohl JJ, Perleth M, Gartlehner G, Kaminski-Hartenthaler A, Schünemann H. GRADE guidelines: 1. Introduction - GRADE evidence profiles and summary of findings tables [in German]. Z Evid Fortbild Qual Gesundhwes. 2012;106(5):357-368. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

