The Plastidic Sugar Transporter pSuT Influences Flowering and Affects Cold Responses
- PMID: 30482788
- PMCID: PMC6426421
- DOI: 10.1104/pp.18.01036
The Plastidic Sugar Transporter pSuT Influences Flowering and Affects Cold Responses
Abstract
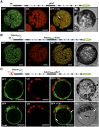
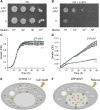
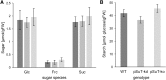
Sucrose (Suc) is one of the most important types of sugars in plants, serving inter alia as a long-distance transport molecule, a carbon and energy storage compound, an osmotically active solute, and fuel for many anabolic reactions. Suc biosynthesis and degradation pathways are well known; however, the regulation of Suc intracellular distribution is poorly understood. In particular, the cellular function of chloroplast Suc reserves and the transporters involved in accumulating these substantial Suc levels remain uncharacterized. Here, we characterize the plastidic sugar transporter (pSuT) in Arabidopsis (Arabidopsis thaliana), which belongs to a subfamily of the monosaccharide transporter-like family. Transport analyses with yeast cells expressing a truncated, vacuole-targeted version of pSuT indicate that both glucose and Suc act as substrates, and nonaqueous fractionation supports a role for pSuT in Suc export from the chloroplast. The latter process is required for a correct transition from vegetative to reproductive growth and influences inflorescence architecture. Moreover, pSuT activity affects freezing-induced electrolyte release. These data further underline the central function of the chloroplast for plant development and the modulation of stress tolerance.
© 2019 American Society of Plant Biologists. All Rights Reserved.
Figures







Similar articles
-
Proton-driven sucrose symport and antiport are provided by the vacuolar transporters SUC4 and TMT1/2.Plant J. 2011 Oct;68(1):129-36. doi: 10.1111/j.1365-313X.2011.04672.x. Epub 2011 Jul 27. Plant J. 2011. PMID: 21668536
-
Chloroplasts Are Central Players in Sugar-Induced Leaf Growth.Plant Physiol. 2016 May;171(1):590-605. doi: 10.1104/pp.15.01669. Epub 2016 Mar 1. Plant Physiol. 2016. PMID: 26932234 Free PMC article.
-
Overexpression of the vacuolar sugar carrier AtSWEET16 modifies germination, growth, and stress tolerance in Arabidopsis.Plant Physiol. 2013 Nov;163(3):1338-52. doi: 10.1104/pp.113.224972. Epub 2013 Sep 12. Plant Physiol. 2013. PMID: 24028846 Free PMC article.
-
The monosaccharide transporter(-like) gene family in Arabidopsis.FEBS Lett. 2007 May 25;581(12):2318-24. doi: 10.1016/j.febslet.2007.03.016. Epub 2007 Mar 15. FEBS Lett. 2007. PMID: 17379213 Review.
-
Cold stress signalling in female reproductive tissues.Plant Cell Environ. 2019 Mar;42(3):846-853. doi: 10.1111/pce.13408. Epub 2018 Aug 21. Plant Cell Environ. 2019. PMID: 30043473 Review.
Cited by
-
Emerging Roles of SWEET Sugar Transporters in Plant Development and Abiotic Stress Responses.Cells. 2022 Apr 12;11(8):1303. doi: 10.3390/cells11081303. Cells. 2022. PMID: 35455982 Free PMC article. Review.
-
Transcriptome Analysis Reveals Roles of Anthocyanin- and Jasmonic Acid-Biosynthetic Pathways in Rapeseed in Response to High Light Stress.Int J Mol Sci. 2021 Dec 1;22(23):13027. doi: 10.3390/ijms222313027. Int J Mol Sci. 2021. PMID: 34884828 Free PMC article.
-
Transcriptome profiling based on Illumina- and SMRT-based RNA-seq reveals circadian regulation of key pathways in flower bud development in walnut.PLoS One. 2021 Nov 18;16(11):e0260017. doi: 10.1371/journal.pone.0260017. eCollection 2021. PLoS One. 2021. PMID: 34793486 Free PMC article.
-
Genome-Wide Identification and Expression Patterns of Cucumber Invertases and Their Inhibitor Genes.Int J Mol Sci. 2023 Aug 30;24(17):13421. doi: 10.3390/ijms241713421. Int J Mol Sci. 2023. PMID: 37686228 Free PMC article.
-
Dynamics of Plant Metabolism during Cold Acclimation.Int J Mol Sci. 2019 Oct 30;20(21):5411. doi: 10.3390/ijms20215411. Int J Mol Sci. 2019. PMID: 31671650 Free PMC article. Review.
References
-
- Alberdi M, Corcuera LJ (1991) Cold acclimation in plants. Phytochemistry 30: 3177–3184
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

