Mycobacterium tuberculosis Type VII Secretion System Effectors Differentially Impact the ESCRT Endomembrane Damage Response
- PMID: 30482832
- PMCID: PMC6282207
- DOI: 10.1128/mBio.01765-18
Mycobacterium tuberculosis Type VII Secretion System Effectors Differentially Impact the ESCRT Endomembrane Damage Response
Abstract
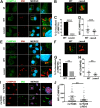
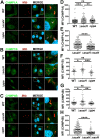
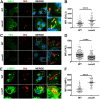
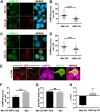
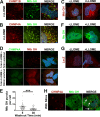
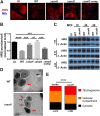
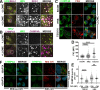
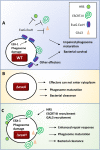
Intracellular pathogens have varied strategies to breach the endolysosomal barrier so that they can deliver effectors to the host cytosol, access nutrients, replicate in the cytoplasm, and avoid degradation in the lysosome. In the case of Mycobacterium tuberculosis, the bacterium perforates the phagosomal membrane shortly after being taken up by macrophages. Phagosomal damage depends upon the mycobacterial ESX-1 type VII secretion system (T7SS). Sterile insults, such as silica crystals or membranolytic peptides, can also disrupt phagosomal and endolysosomal membranes. Recent work revealed that the host endosomal sorting complex required for transport (ESCRT) machinery rapidly responds to sterile endolysosomal damage and promotes membrane repair. We hypothesized that ESCRTs might also respond to pathogen-induced phagosomal damage and that M. tuberculosis could impair this host response. Indeed, we found that ESCRT-III proteins were recruited to M. tuberculosis phagosomes in an ESX-1-dependent manner. We previously demonstrated that the mycobacterial effectors EsxG/TB9.8 and EsxH/TB10.4, both secreted by the ESX-3 T7SS, can inhibit ESCRT-dependent trafficking of receptors to the lysosome. Here, we additionally show that ESCRT-III recruitment to sites of endolysosomal damage is antagonized by EsxG and EsxH, both within the context of M. tuberculosis infection and sterile injury. Moreover, EsxG and EsxH themselves respond within minutes to membrane damage in a manner that is independent of calcium and ESCRT-III recruitment. Thus, our study reveals that T7SS effectors and ESCRT participate in a series of measures and countermeasures for control of phagosome integrity.IMPORTANCEMycobacterium tuberculosis causes tuberculosis, which kills more people than any other infection. M. tuberculosis grows in macrophages, cells that specialize in engulfing and degrading microorganisms. Like many intracellular pathogens, in order to cause disease, M. tuberculosis damages the membrane-bound compartment (phagosome) in which it is enclosed after macrophage uptake. Recent work showed that when chemicals damage this type of intracellular compartment, cells rapidly detect and repair the damage, using machinery called the endosomal sorting complex required for transport (ESCRT). Therefore, we hypothesized that ESCRT might also respond to pathogen-induced damage. At the same time, our previous work showed that the EsxG-EsxH heterodimer of M. tuberculosis can inhibit ESCRT, raising the possibility that M. tuberculosis impairs this host response. Here, we show that ESCRT is recruited to damaged M. tuberculosis phagosomes and that EsxG-EsxH undermines ESCRT-mediated endomembrane repair. Thus, our studies demonstrate a battle between host and pathogen over endomembrane integrity.
Keywords: ESCRT; Mycobacterium tuberculosis; endomembrane damage; phagosomes; type VII secretion system.
Copyright © 2018 Mittal et al.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

