The Widely Conserved ebo Cluster Is Involved in Precursor Transport to the Periplasm during Scytonemin Synthesis in Nostoc punctiforme
- PMID: 30482833
- PMCID: PMC6282210
- DOI: 10.1128/mBio.02266-18
The Widely Conserved ebo Cluster Is Involved in Precursor Transport to the Periplasm during Scytonemin Synthesis in Nostoc punctiforme
Abstract
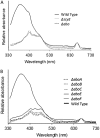
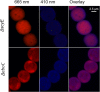
Scytonemin is a dimeric indole-phenol sunscreen synthesized by some cyanobacteria under conditions of exposure to UVA radiation. While its biosynthetic pathway has been elucidated only partially, comparative genomics reveals that the scytonemin operon often contains a cluster of five highly conserved genes (ebo cluster) of unknown function that is widespread and conserved among several bacterial and algal phyla. We sought to elucidate the function of the ebo cluster in the cyanobacterium Nostoc punctiforme by constructing and analyzing in-frame deletion mutants (one for each ebo gene and one for the entire cluster). Under conditions of UVA induction, all ebo mutants were scytoneminless, and all accumulated a single compound, the scytonemin monomer, clearly implicating all ebo genes in scytonemin production. We showed that the scytonemin monomer also accumulated in an induced deletion mutant of scyE, a non-ebo scytonemin gene whose product is demonstrably targeted to the periplasm. Confocal autofluorescence microscopy revealed that the accumulation was confined to the cytoplasm in all ebo mutants but that that was not the case in the scyE deletion, with an intact ebo cluster, where the scytonemin monomer was also excreted to the periplasm. The results implicate the ebo cluster in the export of the scytonemin monomer to the periplasm for final oxidative dimerization by ScyE. By extension, the ebo gene cluster may play similar roles in metabolite translocation across many bacterial phyla. We discuss potential mechanisms for such a role on the basis of structural and phylogenetic considerations of the ebo proteins.IMPORTANCE Elucidating the biochemical and genetic basis of scytonemin constitutes an interesting challenge because of its unique structure and the unusual fact that it is partially synthesized in the periplasmic space. Our work points to the ebo gene cluster, associated with the scytonemin operon of cyanobacteria, as being responsible for the excretion of scytonemin intermediates from the cytoplasm into the periplasm during biosynthesis. Few conserved systems have been described that facilitate the membrane translocation of small molecules. Because the ebo cluster is well conserved among a large diversity of bacteria and algae and yet insights into its potential function are lacking, our findings suggest that translocation of small molecules across the plasma membrane may be its generic role across microbes.
Keywords: alkaloids; cyanobacteria; ebo genes; excretion; lipid carriers; membrane transport; periplasm; scytonemin; secondary metabolism; sunscreens.
Copyright © 2018 Klicki et al.
Figures





Similar articles
-
A comparative genomics approach to understanding the biosynthesis of the sunscreen scytonemin in cyanobacteria.BMC Genomics. 2009 Jul 24;10:336. doi: 10.1186/1471-2164-10-336. BMC Genomics. 2009. PMID: 19630972 Free PMC article.
-
Molecular genetics and genomic analysis of scytonemin biosynthesis in Nostoc punctiforme ATCC 29133.J Bacteriol. 2007 Jun;189(12):4465-72. doi: 10.1128/JB.01816-06. Epub 2007 Mar 9. J Bacteriol. 2007. PMID: 17351042 Free PMC article.
-
Extracellular Polysaccharide Production in a Scytonemin-Deficient Mutant of Nostoc punctiforme Under UVA and Oxidative Stress.Curr Microbiol. 2016 Oct;73(4):455-62. doi: 10.1007/s00284-016-1084-y. Epub 2016 Jun 15. Curr Microbiol. 2016. PMID: 27301251
-
Biotechnological Production of the Sunscreen Pigment Scytonemin in Cyanobacteria: Progress and Strategy.Mar Drugs. 2021 Feb 27;19(3):129. doi: 10.3390/md19030129. Mar Drugs. 2021. PMID: 33673485 Free PMC article. Review.
-
Cyanobacterial Sunscreen Scytonemin: Role in Photoprotection and Biomedical Research.Appl Biochem Biotechnol. 2015 Jul;176(6):1551-63. doi: 10.1007/s12010-015-1676-1. Epub 2015 May 27. Appl Biochem Biotechnol. 2015. PMID: 26013282 Review.
Cited by
-
Cyanoexosortase B is essential for motility, biofilm formation, and scytonemin production in a filamentous cyanobacterium.mSphere. 2025 Jun 25;10(6):e0100624. doi: 10.1128/msphere.01006-24. Epub 2025 May 13. mSphere. 2025. PMID: 40358234 Free PMC article.
-
EDB Gene Cluster-Dependent Indole Production Is Responsible for the Ability of Pseudomonas fluorescens NZI7 to Repel Grazing by Caenorhabditis elegans.J Nat Prod. 2022 Mar 25;85(3):590-598. doi: 10.1021/acs.jnatprod.1c01046. Epub 2022 Jan 25. J Nat Prod. 2022. PMID: 35077157 Free PMC article.
-
2-Deoxy-4-epi-scyllo-inosose (DEI) is the Product of EboD, a Highly Conserved Dehydroquinate Synthase-like Enzyme in Bacteria and Eustigmatophyte Algae.ACS Chem Biol. 2024 Nov 15;19(11):2277-2283. doi: 10.1021/acschembio.4c00510. Epub 2024 Oct 15. ACS Chem Biol. 2024. PMID: 39404639
-
Multiple concurrent and convergent stages of genome reduction in bacterial symbionts across a stink bug family.Sci Rep. 2021 Apr 8;11(1):7731. doi: 10.1038/s41598-021-86574-8. Sci Rep. 2021. PMID: 33833268 Free PMC article.
-
Plastid Genomes and Proteins Illuminate the Evolution of Eustigmatophyte Algae and Their Bacterial Endosymbionts.Genome Biol Evol. 2019 Feb 1;11(2):362-379. doi: 10.1093/gbe/evz004. Genome Biol Evol. 2019. PMID: 30629162 Free PMC article.
References
-
- Garcia-Pichel F, Castenholz RW. 1991. Characterization and biological implications of scytonemin, a cyanobacterial sheath pigment. J Phycol 27:395–409. doi:10.1111/j.0022-3646.1991.00395.x. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

