Origins and Evolution of the Global RNA Virome
- PMID: 30482837
- PMCID: PMC6282212
- DOI: 10.1128/mBio.02329-18
Origins and Evolution of the Global RNA Virome
Abstract
Viruses with RNA genomes dominate the eukaryotic virome, reaching enormous diversity in animals and plants. The recent advances of metaviromics prompted us to perform a detailed phylogenomic reconstruction of the evolution of the dramatically expanded global RNA virome. The only universal gene among RNA viruses is the gene encoding the RNA-dependent RNA polymerase (RdRp). We developed an iterative computational procedure that alternates the RdRp phylogenetic tree construction with refinement of the underlying multiple-sequence alignments. The resulting tree encompasses 4,617 RNA virus RdRps and consists of 5 major branches; 2 of the branches include positive-sense RNA viruses, 1 is a mix of positive-sense (+) RNA and double-stranded RNA (dsRNA) viruses, and 2 consist of dsRNA and negative-sense (-) RNA viruses, respectively. This tree topology implies that dsRNA viruses evolved from +RNA viruses on at least two independent occasions, whereas -RNA viruses evolved from dsRNA viruses. Reconstruction of RNA virus evolution using the RdRp tree as the scaffold suggests that the last common ancestors of the major branches of +RNA viruses encoded only the RdRp and a single jelly-roll capsid protein. Subsequent evolution involved independent capture of additional genes, in particular, those encoding distinct RNA helicases, enabling replication of larger RNA genomes and facilitating virus genome expression and virus-host interactions. Phylogenomic analysis reveals extensive gene module exchange among diverse viruses and horizontal virus transfer between distantly related hosts. Although the network of evolutionary relationships within the RNA virome is bound to further expand, the present results call for a thorough reevaluation of the RNA virus taxonomy.IMPORTANCE The majority of the diverse viruses infecting eukaryotes have RNA genomes, including numerous human, animal, and plant pathogens. Recent advances of metagenomics have led to the discovery of many new groups of RNA viruses in a wide range of hosts. These findings enable a far more complete reconstruction of the evolution of RNA viruses than was attainable previously. This reconstruction reveals the relationships between different Baltimore classes of viruses and indicates extensive transfer of viruses between distantly related hosts, such as plants and animals. These results call for a major revision of the existing taxonomy of RNA viruses.
Keywords: RNA virus; RNA-dependent RNA polymerase; capsid protein; evolution; virome.
Figures
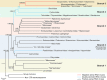




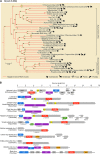


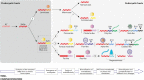
Comment in
-
Can Sequence Phylogenies Safely Infer the Origin of the Global Virome?mBio. 2019 Apr 16;10(2):e00289-19. doi: 10.1128/mBio.00289-19. mBio. 2019. PMID: 30992348 Free PMC article. No abstract available.
-
Reply to Holmes and Duchêne, "Can Sequence Phylogenies Safely Infer the Origin of the Global Virome?": Deep Phylogenetic Analysis of RNA Viruses Is Highly Challenging but Not Meaningless.mBio. 2019 Apr 16;10(2):e00542-19. doi: 10.1128/mBio.00542-19. mBio. 2019. PMID: 30992352 Free PMC article. No abstract available.
References
-
- Gilbert W. 1986. Origin of life - the RNA world. Nature 319:618–618. doi: 10.1038/319618a0. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
