Cooperative actions of Tbc1d1 and AS160/Tbc1d4 in GLUT4-trafficking activities
- PMID: 30482843
- PMCID: PMC6349102
- DOI: 10.1074/jbc.RA118.004614
Cooperative actions of Tbc1d1 and AS160/Tbc1d4 in GLUT4-trafficking activities
Abstract
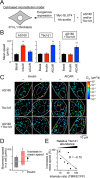
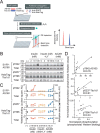
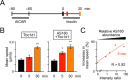
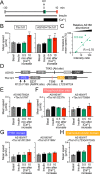
AS160 and Tbc1d1 are key Rab GTPase-activating proteins (RabGAPs) that mediate release of static GLUT4 in response to insulin or exercise-mimetic stimuli, respectively, but their cooperative regulation and its underlying mechanisms remain unclear. By employing GLUT4 nanometry with cell-based reconstitution models, we herein analyzed the functional cooperative activities of the RabGAPs. When both RabGAPs are present, Tbc1d1 functionally dominates AS160, and stimuli-inducible GLUT4 release relies on Tbc1d1-evoking proximal stimuli, such as AICAR and intracellular Ca2+ Detailed functional assessments with varying expression ratios revealed that AS160 modulates sensitivity to external stimuli in Tbc1d1-mediated GLUT4 release. For example, Tbc1d1-governed GLUT4 release triggered by Ca2+ plus insulin occurred more efficiently than that in cells with little or no AS160. Series of mutational analyses revealed that these synergizing actions rely on the phosphotyrosine-binding 1 (PTB1) and calmodulin-binding domains of Tbc1d1 as well as key phosphorylation sites of both AS160 (Thr642) and Tbc1d1 (Ser237 and Thr596). Thus, the emerging cooperative governance relying on the multiple regulatory nodes of both Tbc1d1 and AS160, functioning together, plays a key role in properly deciphering biochemical signals into a physical GLUT4 release process in response to insulin, exercise, and the two in combination.
Keywords: AMP-activated kinase (AMPK); Akt PKB; GTPase activating protein (GAP); glucose transporter type 4 (GLUT4); insulin; membrane trafficking; microscopic imaging; signal transduction.
© 2019 Hatakeyama et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
-
- Taylor E. B., An D., Kramer H. F., Yu H., Fujii N. L., Roeckl K. S., Bowles N., Hirshman M. F., Xie J., Feener E. P., and Goodyear L. J. (2008) Discovery of TBC1D1 as an insulin-, AICAR-, and contraction-stimulated signaling nexus in mouse skeletal muscle. J. Biol. Chem. 283, 9787–9796 10.1074/jbc.M708839200 - DOI - PMC - PubMed
-
- Kane S., Sano H., Liu S. C., Asara J. M., Lane W. S., Garner C. C., and Lienhard G. E. (2002) A method to identify serine kinase substrates: Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J. Biol. Chem. 277, 22115–22118 10.1074/jbc.C200198200 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

