Surface Loops in a Single SH2 Domain Are Capable of Encoding the Spectrum of Specificity of the SH2 Family
- PMID: 30482845
- PMCID: PMC6356082
- DOI: 10.1074/mcp.RA118.001123
Surface Loops in a Single SH2 Domain Are Capable of Encoding the Spectrum of Specificity of the SH2 Family
Abstract
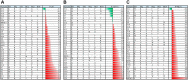
Src homology 2 (SH2) domains play an essential role in cellular signal transduction by binding to proteins phosphorylated on Tyr residue. Although Tyr phosphorylation (pY) is a prerequisite for binding for essentially all SH2 domains characterized to date, different SH2 domains prefer specific sequence motifs C-terminal to the pY residue. Because all SH2 domains adopt the same structural fold, it is not well understood how different SH2 domains have acquired the ability to recognize distinct sequence motifs. We have shown previously that the EF and BG loops that connect the secondary structure elements on an SH2 domain dictate its specificity. In this study, we investigated if these surface loops could be engineered to encode diverse specificities. By characterizing a group of SH2 variants selected by different pY peptides from phage-displayed libraries, we show that the EF and BG loops of the Fyn SH2 domain can encode a wide spectrum of specificities, including all three major specificity classes (p + 2, p + 3 and p + 4) of the SH2 domain family. Furthermore, we found that the specificity of a given variant correlates with the sequence feature of the bait peptide used for its isolation, suggesting that an SH2 domain may acquire specificity by co-evolving with its ligand. Intriguingly, we found that the SH2 variants can employ a variety of different mechanisms to confer the same specificity, suggesting the EF and BG loops are highly flexible and adaptable. Our work provides a plausible mechanism for the SH2 domain to acquire the wide spectrum of specificity observed in nature through loop variation with minimal disturbance to the SH2 fold. It is likely that similar mechanisms may have been employed by other modular interaction domains to generate diversity in specificity.
Keywords: Antibiotics; Autophagy; Breast Cancer; Cancer Biology; Cancer Stem Cells; Chemoresistance; Clinical Proteomics; Mitochondria; Mitochondria Function or Biology; NMR; NMR-metabolomic; Stem Cells.
© 2019 Liu et al.
Figures


References
-
- Pawson T., and Scott J. D. (2005) Protein phosphorylation in signaling–50 years and counting. Trends Biochem. Sci. 30, 286–290 - PubMed
-
- Huang H., Li L., Wu C., Schibli D., Colwill K., Ma S., Li C., Roy P., Ho K., Songyang Z., Pawson T., Gao Y., and Li S.S. (2008) Defining the specificity space of the human SRC homology 2 domain. Mol. Cell. Proteomics 7, 768–784 - PubMed
-
- Liu B. A., Jablonowski K., Raina M., Arcé M., Pawson T., and Nash P.D. (2006) The human and mouse complement of SH2 domain proteins establishing the boundaries of phosphotyrosine signaling. Mol. Cell 22, 851–868 - PubMed
-
- Pawson T. (2004) Specificity in signal transduction: from phosphotyrosine-SH2 domain interactions to complex cellular systems. Cell 116, 191–203 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

