Dynamic Phosphoproteomics Uncovers Signaling Pathways Modulated by Anti-oncogenic Sphingolipid Analogs
- PMID: 30482847
- PMCID: PMC6398214
- DOI: 10.1074/mcp.RA118.001053
Dynamic Phosphoproteomics Uncovers Signaling Pathways Modulated by Anti-oncogenic Sphingolipid Analogs
Abstract
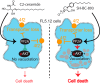
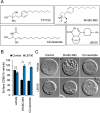
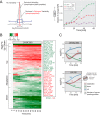
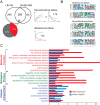
The anti-neoplastic sphingolipid analog SH-BC-893 starves cancer cells to death by down-regulating cell surface nutrient transporters and blocking lysosomal trafficking events. These effects are mediated by the activation of protein phosphatase 2A (PP2A). To identify putative PP2A substrates, we used quantitative phosphoproteomics to profile the temporal changes in protein phosphorylation in FL5.12 cells following incubation with SH-BC-893 or the specific PP2A inhibitor LB-100. These analyses enabled the profiling of more than 15,000 phosphorylation sites, of which 958 sites on 644 proteins were dynamically regulated. We identified 114 putative PP2A substrates including several nutrient transporter proteins, GTPase regulators (e.g. Agap2, Git1), and proteins associated with actin cytoskeletal remodeling (e.g. Vim, Pxn). To identify SH-BC-893-induced cell signaling events that disrupt lysosomal trafficking, we compared phosphorylation profiles in cells treated with SH-BC-893 or C2-ceramide, a non-vacuolating sphingolipid that does not impair lysosomal fusion. These analyses combined with functional assays uncovered the differential regulation of Akt and Gsk3b by SH-BC-893 (vacuolating) and C2-ceramide (non-vacuolating). Dynamic phosphoproteomics of cells treated with compounds affecting PP2A activity thus enabled the correlation of cell signaling with phenotypes to rationalize their mode of action.
Keywords: Cancer Biology*; Cell biology*; Phosphoproteome; Phosphorylation; Quantification.
© 2019 Kubiniok et al.
Conflict of interest statement
We declare no competing financial interests
Figures


Similar articles
-
Targeting cancer metabolism by simultaneously disrupting parallel nutrient access pathways.J Clin Invest. 2016 Nov 1;126(11):4088-4102. doi: 10.1172/JCI87148. Epub 2016 Sep 26. J Clin Invest. 2016. PMID: 27669461 Free PMC article.
-
Phosphoproteomics Profiling of Nonsmall Cell Lung Cancer Cells Treated with a Novel Phosphatase Activator.Proteomics. 2017 Nov;17(22):10.1002/pmic.201700214. doi: 10.1002/pmic.201700214. Epub 2017 Oct 25. Proteomics. 2017. PMID: 28961369 Free PMC article.
-
PP2A inhibition by LB-100 protects retinal pigment epithelium cells from UV radiation via activation of AMPK signaling.Biochem Biophys Res Commun. 2018 Nov 17;506(1):73-80. doi: 10.1016/j.bbrc.2018.10.077. Epub 2018 Oct 16. Biochem Biophys Res Commun. 2018. PMID: 30340831
-
LB100, a small molecule inhibitor of PP2A with potent chemo- and radio-sensitizing potential.Cancer Biol Ther. 2015;16(6):821-33. doi: 10.1080/15384047.2015.1040961. Epub 2015 Apr 21. Cancer Biol Ther. 2015. PMID: 25897893 Free PMC article. Review.
-
Quantitative phosphoproteomics to characterize signaling networks.Semin Cell Dev Biol. 2012 Oct;23(8):863-71. doi: 10.1016/j.semcdb.2012.05.006. Epub 2012 Jun 5. Semin Cell Dev Biol. 2012. PMID: 22677334 Review.
Cited by
-
Synthetic Sphingolipids with 1,2-Pyridazine Appendages Improve Antiproliferative Activity in Human Cancer Cell Lines.ACS Med Chem Lett. 2020 Feb 12;11(5):686-690. doi: 10.1021/acsmedchemlett.9b00553. eCollection 2020 May 14. ACS Med Chem Lett. 2020. PMID: 32435371 Free PMC article.
-
Phosphoproteomics: a valuable tool for uncovering molecular signaling in cancer cells.Expert Rev Proteomics. 2021 Aug;18(8):661-674. doi: 10.1080/14789450.2021.1976152. Epub 2021 Sep 16. Expert Rev Proteomics. 2021. PMID: 34468274 Free PMC article. Review.
-
Phosphoproteomic Approaches for Identifying Phosphatase and Kinase Substrates.Molecules. 2023 Apr 24;28(9):3675. doi: 10.3390/molecules28093675. Molecules. 2023. PMID: 37175085 Free PMC article. Review.
-
Simultaneous inhibition of endocytic recycling and lysosomal fusion sensitizes cells and tissues to oligonucleotide therapeutics.Nucleic Acids Res. 2023 Feb 28;51(4):1583-1599. doi: 10.1093/nar/gkad023. Nucleic Acids Res. 2023. PMID: 36727438 Free PMC article.
-
Design, synthesis and anticancer activity of constrained sphingolipid-phenoxazine/phenothiazine hybrid constructs targeting protein phosphatase 2A.Bioorg Med Chem Lett. 2019 Sep 15;29(18):2681-2685. doi: 10.1016/j.bmcl.2019.07.023. Epub 2019 Jul 19. Bioorg Med Chem Lett. 2019. PMID: 31383588 Free PMC article.
References
-
- Hyde R., Hajduch E., Powell D. J., Taylor P. M., and Hundal H. S. (2005) Ceramide down-regulates System A amino acid transport and protein synthesis in rat skeletal muscle cells. FASEB J. 19, 461–463 - PubMed
-
- Kim S. M., Roy S. G., Chen B., Nguyen T. M., McMonigle R. J., McCracken A. N., Zhang Y., Kofuji S., Hou J., Selwan E., Finicle B. T., Nguyen T. T., Ravi A., Ramirez M. U., Wiher T., Guenther G. G., Kono M., Sasaki A. T., Weisman L. S., Potma E. O., Tromberg B. J., Edwards R. A., Hanessian S., and Edinger A. L. (2016) Targeting cancer metabolism by simultaneously disrupting parallel nutrient access pathways. J. Clin. Invest. 126, 4088–4102 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

