Transforming growth factor-β1 up-regulates connexin43 expression in osteocytes via canonical Smad-dependent signaling pathway
- PMID: 30482881
- PMCID: PMC6294634
- DOI: 10.1042/BSR20181678
Transforming growth factor-β1 up-regulates connexin43 expression in osteocytes via canonical Smad-dependent signaling pathway
Erratum in
-
Correction: Transforming growth factor-β1 up-regulates connexin43 expression in osteocytes via canonical Smad-dependent signaling pathway.Biosci Rep. 2020 Oct 30;40(10):BSR-20181678_COR. doi: 10.1042/BSR-20181678_COR. Biosci Rep. 2020. PMID: 33063831 Free PMC article. No abstract available.
Expression of concern in
-
Expression of Concern: Transforming growth factor-β1 up-regulates connexin43 expression in osteocytes via canonical Smad-dependent signaling pathway.Biosci Rep. 2020 Jul 31;40(7):BSR-20181678_EOC. doi: 10.1042/BSR-20181678_EOC. Biosci Rep. 2020. PMID: 32725121 Free PMC article. No abstract available.
Abstract
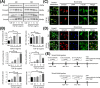
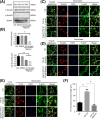
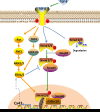
Connexin 43 (Cx43)-mediated gap junctional intercellular communication (GJIC) has been shown to be important in regulating multiple functions of bone cells. Transforming growth factor-β1 (TGF-β1) exhibited controversial effects on the expression of Cx43 in different cell types. To date, the effect of TGF-β1 on the Cx43 expression of osteocytes is still unknown. In the present study, we detected the expression of TGF-β1 in osteocytes and bone tissue, and then used recombinant mouse TGF-β1 to elucidate its effect on gap junctions (GJs) of osteocytes. Our data indicated that TGF-β1 up-regulated both mRNA and protein expression of Cx43 in osteocytes. Together with down-regulation of Cx43 expression after being treated with TGF-β type I receptor inhibitor Repsox, we deduced that TGF-β1 can positively regulate Cx43 expression in osteocytes. Thus we next focussed on the downstream signals of TGF-β and found that TGF-β1-mediated smads, Smad3 and Smad4, to translocate into nucleus. These translocated signal proteins bind to the promoter of Gja1 which was responsible for the changed expression of Cx43. The present study provides evidence that TGF-β1 can enhance GJIC between osteocytes through up-regulating Cx43 expression and the underlying mechanism involved in the activation of Smad-dependent pathway.
Keywords: Connexin 43; Gap junction; Osteocyte; Smad3; Smad4; TGF-β1.
© 2018 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures
Similar articles
-
Transforming growth factor-β1 up-regulates connexin43 expression in human granulosa cells.Hum Reprod. 2015 Sep;30(9):2190-201. doi: 10.1093/humrep/dev175. Epub 2015 Jul 22. Hum Reprod. 2015. PMID: 26202915 Free PMC article.
-
The involvement of the ERK-MAPK pathway in TGF-β1-mediated connexin43-gap junction formation in chondrocytes.Connect Tissue Res. 2019 Sep;60(5):477-486. doi: 10.1080/03008207.2019.1593394. Epub 2019 Mar 22. Connect Tissue Res. 2019. PMID: 30897973
-
TGF-β1 promotes gap junctions formation in chondrocytes via Smad3/Smad4 signalling.Cell Prolif. 2019 Mar;52(2):e12544. doi: 10.1111/cpr.12544. Epub 2018 Nov 15. Cell Prolif. 2019. PMID: 30444057 Free PMC article.
-
Gap junctional regulation of signal transduction in bone cells.FEBS Lett. 2014 Apr 17;588(8):1315-21. doi: 10.1016/j.febslet.2014.01.025. Epub 2014 Jan 28. FEBS Lett. 2014. PMID: 24486014 Free PMC article. Review.
-
Shifting paradigms on the role of connexin43 in the skeletal response to mechanical load.J Bone Miner Res. 2014 Feb;29(2):275-86. doi: 10.1002/jbmr.2165. J Bone Miner Res. 2014. PMID: 24588015 Free PMC article. Review.
Cited by
-
[Metformin inhibits collagen production in rat biliary fibroblasts: the molecular signaling mechanism].Nan Fang Yi Ke Da Xue Xue Bao. 2020 May 30;40(5):640-646. doi: 10.12122/j.issn.1673-4254.2020.05.05. Nan Fang Yi Ke Da Xue Xue Bao. 2020. PMID: 32897197 Free PMC article. Chinese.
-
A "Connexin" Responsible for the Fatal Attraction of Cancer to Bone.Cell Metab. 2019 Jan 8;29(1):6-8. doi: 10.1016/j.cmet.2018.12.014. Cell Metab. 2019. PMID: 30625309 Free PMC article.
-
Blocking Connexin-43 mediated hemichannel activity protects against early tubular injury in experimental chronic kidney disease.Cell Commun Signal. 2020 May 25;18(1):79. doi: 10.1186/s12964-020-00558-1. Cell Commun Signal. 2020. PMID: 32450899 Free PMC article.
-
TGF-β1 facilitates cell-cell communication in osteocytes via connexin43- and pannexin1-dependent gap junctions.Cell Death Discov. 2019 Oct 25;5:141. doi: 10.1038/s41420-019-0221-3. eCollection 2019. Cell Death Discov. 2019. PMID: 31666990 Free PMC article.
-
The Smad Dependent TGF-β and BMP Signaling Pathway in Bone Remodeling and Therapies.Front Mol Biosci. 2021 May 5;8:593310. doi: 10.3389/fmolb.2021.593310. eCollection 2021. Front Mol Biosci. 2021. PMID: 34026818 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

