TGF-β induces corneal endothelial senescence via increase of mitochondrial reactive oxygen species in chronic corneal allograft failure
- PMID: 30482886
- PMCID: PMC6286827
- DOI: 10.18632/aging.101659
TGF-β induces corneal endothelial senescence via increase of mitochondrial reactive oxygen species in chronic corneal allograft failure
Abstract
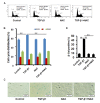
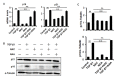
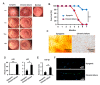
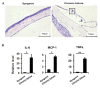
The corneal endothelium (CE) dysfunction impairs optical transparency and leads to corneal allograft failure. Morphologically, CE cells are characterized by premature senescence at the late stage of corneal graft. However, the detailed molecular mechanisms are largely unknown. Here we found that transforming growth factor-β (TGF-β) is elevated in the CE of late graft failure. In addition, senescence-associated gene p21 and p16 are increased as well, which is consistent with their elevation upon TGF-β treatment in human corneal endothelial cell B4G12. Furthermore, TGF-β treatment leads to high positive ratio of SA-β-gal, indicating B4G12 cells undergo cellular senescence. Mechanistically, we demonstrated that TGF-β could induce mitochondrial ROS (mtROS) production and mtROS scavenger could rescue CE cell senescence upon TGF-β treatment. Our study provides new evidence that elevated TGF-β plays a crucial role in the CE cell senescence and loss in chronic corneal graft failure, which could be potential targets for clinical treatment.
Keywords: cellular senescence; chronic corneal graft failure; corneal endothelium; mitochondrial reactive oxygen species; transforming growth factor-β.
Conflict of interest statement
Figures


Similar articles
-
TGF-β and NF-κB signaling pathway crosstalk potentiates corneal epithelial senescence through an RNA stress response.Aging (Albany NY). 2016 Oct 6;8(10):2337-2354. doi: 10.18632/aging.101050. Aging (Albany NY). 2016. PMID: 27713146 Free PMC article.
-
TGF-β1 induces senescence of bone marrow mesenchymal stem cells via increase of mitochondrial ROS production.BMC Dev Biol. 2014 May 18;14:21. doi: 10.1186/1471-213X-14-21. BMC Dev Biol. 2014. PMID: 24886313 Free PMC article.
-
Senolytic Treatment Alleviates Corneal Allograft Rejection Through Upregulation of Angiotensin-Converting Enzyme 2 (ACE2).Invest Ophthalmol Vis Sci. 2025 Feb 3;66(2):15. doi: 10.1167/iovs.66.2.15. Invest Ophthalmol Vis Sci. 2025. PMID: 39913165 Free PMC article.
-
Endothelial mitochondrial senescence accelerates cardiovascular disease in antiretroviral-receiving HIV patients.Toxicol Lett. 2019 Dec 15;317:13-23. doi: 10.1016/j.toxlet.2019.09.018. Epub 2019 Sep 25. Toxicol Lett. 2019. PMID: 31562912 Review.
-
[Transplantation of corneal endothelial cells].Nippon Ganka Gakkai Zasshi. 2002 Dec;106(12):805-35; discussion 836. Nippon Ganka Gakkai Zasshi. 2002. PMID: 12610838 Review. Japanese.
Cited by
-
Squishy matters - Corneal mechanobiology in health and disease.Prog Retin Eye Res. 2024 Mar;99:101234. doi: 10.1016/j.preteyeres.2023.101234. Epub 2024 Jan 2. Prog Retin Eye Res. 2024. PMID: 38176611 Free PMC article. Review.
-
Proliferator-Activated Receptor Alpha Inhibits Abnormal Extracellular Matrix Accumulation and Maintains Energy Metabolism in Late-Onset Fuchs Endothelial Corneal Dystrophy.Invest Ophthalmol Vis Sci. 2025 Apr 1;66(4):36. doi: 10.1167/iovs.66.4.36. Invest Ophthalmol Vis Sci. 2025. PMID: 40232711 Free PMC article.
-
TGF‑β induces periodontal ligament stem cell senescence through increase of ROS production.Mol Med Rep. 2019 Oct;20(4):3123-3130. doi: 10.3892/mmr.2019.10580. Epub 2019 Aug 9. Mol Med Rep. 2019. PMID: 31432132 Free PMC article.
-
Transplantation of human induced pluripotent stem cell-derived neural crest cells for corneal endothelial regeneration.Stem Cell Res Ther. 2021 Mar 29;12(1):214. doi: 10.1186/s13287-021-02267-z. Stem Cell Res Ther. 2021. PMID: 33781330 Free PMC article.
-
A p-Tyr42 RhoA Inhibitor Promotes the Regeneration of Human Corneal Endothelial Cells by Ameliorating Cellular Senescence.Antioxidants (Basel). 2023 May 30;12(6):1186. doi: 10.3390/antiox12061186. Antioxidants (Basel). 2023. PMID: 37371916 Free PMC article.
References
-
- Lass JH, Gal RL, Dontchev M, Beck RW, Kollman C, Dunn SP, Heck E, Holland EJ, Mannis MJ, Montoya MM, Schultze RL, Stulting RD, Sugar A, et al., and Cornea Donor Study Investigator Group. Donor age and corneal endothelial cell loss 5 years after successful corneal transplantation. Specular microscopy ancillary study results. Ophthalmology. 2008; 115:627–632.e8. 10.1016/j.ophtha.2008.01.004 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

