Suppression of mTORC1 activity in senescent Ras-transformed cells neither restores autophagy nor abrogates apoptotic death caused by inhibition of MEK/ERK kinases
- PMID: 30482887
- PMCID: PMC6286832
- DOI: 10.18632/aging.101686
Suppression of mTORC1 activity in senescent Ras-transformed cells neither restores autophagy nor abrogates apoptotic death caused by inhibition of MEK/ERK kinases
Abstract
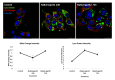
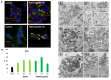
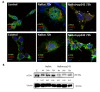
Autophagy is conservative catabolic process that degrades organelles, in particular, mitochondria, and misfolded proteins within the lysosomes, thus maintaining cellular viability. Despite the close relationship between mitochondrial dysfunction and cellular senescence, it is unclear how mitochondria damage can induce autophagy in senescent cells. We show that MEK/ERK suppression induces mitochondria damage followed by apoptosis of senescent Ras-expressing cells. To understand the role of persistent mTORC1 signaling in breaking the cAMPK-induced autophagy caused by mitochondrial damage, we inhibited mTORС1 with low concentrations of pp242. mTORC1 suppression neither restores the AMPK-induced autophagy nor decreases the level of apoptosis upon MEK/ERK inhibition. We discovered the existence of an alternative autophagy-like way that partially increases the viability of senescent cells under suppressed mTORC1. The pp242-treated cells survive due to formation of the non-autophagous LC3-negative vacuoles, which contain the damaged mitochondria and lysosomes with the following excretion the content from the cell. MEK/ERK activity is required to implement this process in senescent cells. Senescent cells exhibit distinctive spatial distribution of organelles and proteins that provides uncoupling of final participants of autophagy. We show that this feature stops the process of cytoprotective autophagy in response to MEK/ERK suppression, thus allowing selective elimination of senescent Ras-expressing cells.
Keywords: MEK/ERK; autophagy; kinase inhibitors; lysosomes; mTOR; mitochondria damage; senescence.
Conflict of interest statement
Figures





References
-
- Hosokawa N, Hara T, Kaizuka T, Kishi C, Takamura A, Miura Y, Iemura S, Natsume T, Takehana K, Yamada N, Guan JL, Oshiro N, Mizushima N. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Nat Cell Biol. 2009; 20:1981–91. 10.1091/mbc.E08-12-1248 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

