Loss of neuronal network resilience precedes seizures and determines the ictogenic nature of interictal synaptic perturbations
- PMID: 30482946
- PMCID: PMC7617160
- DOI: 10.1038/s41593-018-0278-y
Loss of neuronal network resilience precedes seizures and determines the ictogenic nature of interictal synaptic perturbations
Abstract
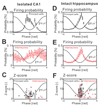
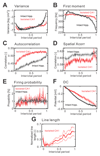
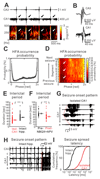
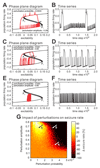
The mechanism of seizure emergence and the role of brief interictal epileptiform discharges (IEDs) in seizure generation are two of the most important unresolved issues in modern epilepsy research. We found that the transition to seizure is not a sudden phenomenon, but is instead a slow process that is characterized by the progressive loss of neuronal network resilience. From a dynamical perspective, the slow transition is governed by the principles of critical slowing, a robust natural phenomenon that is observable in systems characterized by transitions between dynamical regimes. In epilepsy, this process is modulated by synchronous synaptic input from IEDs. IEDs are external perturbations that produce phasic changes in the slow transition process and exert opposing effects on the dynamics of a seizure-generating network, causing either anti-seizure or pro-seizure effects. We found that the multifaceted nature of IEDs is defined by the dynamical state of the network at the moment of the discharge occurrence.
Conflict of interest statement
The authors declare no competing interests.
Figures



Comment in
-
A Straw Can Break a Neural Network's Back and Lead to Seizures-But Only When Delivered at the Right Time.Epilepsy Curr. 2019 Mar-Apr;19(2):115-116. doi: 10.1177/1535759719835349. Epilepsy Curr. 2019. PMID: 30955435 Free PMC article.
References
-
- Fisher RS, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475–482. - PubMed
-
- Lopes da Silva F. In: Seizure Prediction in Epilepsy: From Basic Mechanisms to Clinical Applications. Schelter B, Timmer J, Schulze-Bonhage A, editors. WILEY-VCH Verlag GmbH & Co.; 2008. Epilepsy as a Disease of the Dynamics of Neuronal Networks - Models and Predictions; pp. 97–107.
-
- Beghi E, et al. Recommendation for a definition of acute symptomatic seizure. Epilepsia. 2010;51:671–675. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

