Heat Shock Proteins Regulatory Role in Neurodevelopment
- PMID: 30483047
- PMCID: PMC6244093
- DOI: 10.3389/fnins.2018.00821
Heat Shock Proteins Regulatory Role in Neurodevelopment
Abstract
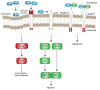
Heat shock proteins (Hsps) are a large family of molecular chaperones that are well-known for their roles in protein maturation, re-folding and degradation. While some Hsps are constitutively expressed in certain regions, others are rapidly upregulated in the presence of stressful stimuli. Numerous stressors, including hyperthermia and hypoxia, can induce the expression of Hsps, which, in turn, interact with client proteins and co-chaperones to regulate cell growth and survival. Such interactions must be tightly regulated, especially at critical points during embryonic and postnatal development. Hsps exhibit specific patterns of expression consistent with a spatio-temporally regulated role in neurodevelopment. There is also growing evidence that Hsps may promote or inhibit neurodevelopment through specific pathways regulating cell differentiation, neurite outgrowth, cell migration, or angiogenesis. This review will examine the regulatory role that these individual chaperones may play in neurodevelopment, and will focus specifically on the signaling pathways involved in the maturation of neuronal and glial cells as well as the underlying vascular network.
Keywords: axon guidance; cell migration; heat shock proteins; neurite extension; neurodevelopment; neurovascular unit.
Figures

Similar articles
-
Molecular chaperones in the kidney: distribution, putative roles, and regulation.Am J Physiol Renal Physiol. 2000 Aug;279(2):F203-15. doi: 10.1152/ajprenal.2000.279.2.F203. Am J Physiol Renal Physiol. 2000. PMID: 10919839 Review.
-
Induction of heat shock proteins and its effects on glial differentiation in rat C6 glioblastoma cells.Kobe J Med Sci. 2001 Apr;47(2):77-95. Kobe J Med Sci. 2001. PMID: 11599126
-
Heat shock proteins in infection.Clin Chim Acta. 2019 Nov;498:90-100. doi: 10.1016/j.cca.2019.08.015. Epub 2019 Aug 19. Clin Chim Acta. 2019. PMID: 31437446 Review.
-
Heat Shock Proteins: Agents of Cancer Development and Therapeutic Targets in Anti-Cancer Therapy.Cells. 2019 Dec 24;9(1):60. doi: 10.3390/cells9010060. Cells. 2019. PMID: 31878360 Free PMC article. Review.
-
Chaperoning STAT3/5 by Heat Shock Proteins: Interest of Their Targeting in Cancer Therapy.Cancers (Basel). 2019 Dec 19;12(1):21. doi: 10.3390/cancers12010021. Cancers (Basel). 2019. PMID: 31861612 Free PMC article. Review.
Cited by
-
Identification of a Thyroid Hormone Binding Site in Hsp90 with Implications for Its Interaction with Thyroid Hormone Receptor Beta.ACS Omega. 2022 Aug 9;7(33):28932-28945. doi: 10.1021/acsomega.2c02331. eCollection 2022 Aug 23. ACS Omega. 2022. PMID: 36033668 Free PMC article.
-
Identification and characterization of proteins that form the inner core Ixodes scapularis tick attachment cement layer.Sci Rep. 2022 Dec 9;12(1):21300. doi: 10.1038/s41598-022-24881-4. Sci Rep. 2022. PMID: 36494396 Free PMC article.
-
Understanding Neurodegeneration from a Clinical and Therapeutic Perspective in Early Diabetic Retinopathy.Nutrients. 2022 Feb 14;14(4):792. doi: 10.3390/nu14040792. Nutrients. 2022. PMID: 35215442 Free PMC article. Review.
-
Manipulation of HSP70-SOD1 Expression Modulates SH-SY5Y Differentiation and Susceptibility to Oxidative Stress-Dependent Cell Damage: Involvement in Oxotremorine-M-Mediated Neuroprotective Effects.Antioxidants (Basel). 2023 Mar 10;12(3):687. doi: 10.3390/antiox12030687. Antioxidants (Basel). 2023. PMID: 36978935 Free PMC article.
-
Physiological and proteomic profiles of Trypanosoma brucei rhodesiense parasite isolated from suramin responsive and non-responsive HAT patients in Busoga, Uganda.Int J Parasitol Drugs Drug Resist. 2021 Apr;15:57-67. doi: 10.1016/j.ijpddr.2021.02.001. Epub 2021 Feb 9. Int J Parasitol Drugs Drug Resist. 2021. PMID: 33588295 Free PMC article.
References
-
- Arrigo A. P., Welch W. J. (1987). Characterization and purification of the small 28,000-dalton mammalian heat shock protein. J. Biol. Chem. 262 15359–15369. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

