Developmental Changes in the Inhibition of Glycinergic Synaptic Currents by Niflumic Acid in Hypoglossal Motoneurons
- PMID: 30483054
- PMCID: PMC6243080
- DOI: 10.3389/fnmol.2018.00416
Developmental Changes in the Inhibition of Glycinergic Synaptic Currents by Niflumic Acid in Hypoglossal Motoneurons
Abstract
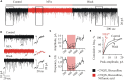
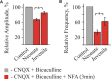
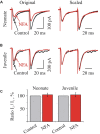
Mammalian brainstem hypoglossal motoneurones (HMs) receive powerful synaptic glycinergic inputs and are involved in a variety of motor functions, including respiration, chewing, sucking, swallowing, and phonation. During the early postnatal development, subunit composition of chloride-permeable glycine receptors (GlyRs) changes leading to a decrease of "fetal" alpha2 and elevation of "adult" alpha1 GlyR subunits. It has been recently demonstrated that niflumic acid (NFA), a member of the fenamate class of non-steroidal anti-inflammatory drugs, is an efficient subunits-specific blocker of GlyRs. At a heterologous expression of different GlyR subunits it has been shown that blocking potency of NFA is more than one order higher for alpha2 GlyRs than for receptors formed by alpha1 subunit. To reveal the action of NFA on the synaptic activity we analyzed here the effects of NFA on the glycinergic inhibitory post-synaptic currents in the HMs from mouse brainstem slices. In the whole-cell patch clamp configuration, the amplitude and the frequency of glycinergic synaptic currents from two age groups have been analyzed: "neonate" (P2-P4) and "juvenile" (P7-P12). Addition of NFA in the presence of antagonists of glutamate and GABA receptors caused a decrease in the mean amplitude and frequency of synaptic events. The degree of the inhibition induced by NFA decreased with the postnatal development, being higher on the motoneurons from "neonate" brainstem slices in comparison with the "juvenile" age group. Analysis of the pair-pulse facilitation suggests the post-synaptic origin of NFA action. These observations provide evidence on the developmental changes in the inhibition by NFA of glycinergic synaptic transmission, which reflects increase in the alpha1 and decrease in the alpha2 GlyR subunits expression in synapses to hypoglossal motoneurons during the early stages of postnatal life.
Keywords: anion-selective channels; brainstem slices; glycine receptors; hypoglossal motoneurons; niflumic acid; patch-clamp recordings.
Figures



Similar articles
-
Voltage-Dependent Inhibition of Glycine Receptor Channels by Niflumic Acid.Front Mol Neurosci. 2017 May 16;10:125. doi: 10.3389/fnmol.2017.00125. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28559795 Free PMC article.
-
Contribution of single-channel properties to the time course and amplitude variance of quantal glycine currents recorded in rat motoneurons.J Neurophysiol. 1999 Apr;81(4):1608-16. doi: 10.1152/jn.1999.81.4.1608. J Neurophysiol. 1999. PMID: 10200197
-
Development of glycinergic synaptic transmission to rat brain stem motoneurons.J Neurophysiol. 1998 Nov;80(5):2608-20. doi: 10.1152/jn.1998.80.5.2608. J Neurophysiol. 1998. PMID: 9819267
-
Development of synaptic transmission to respiratory motoneurons.Respir Physiol Neurobiol. 2011 Oct 15;179(1):34-42. doi: 10.1016/j.resp.2011.03.002. Epub 2011 Mar 5. Respir Physiol Neurobiol. 2011. PMID: 21382524 Free PMC article. Review.
-
Native glycine receptor subtypes and their physiological roles.Neuropharmacology. 2009 Jan;56(1):303-9. doi: 10.1016/j.neuropharm.2008.07.034. Epub 2008 Aug 3. Neuropharmacology. 2009. PMID: 18721822 Review.
Cited by
-
A photoswitchable GABA receptor channel blocker.Br J Pharmacol. 2019 Aug;176(15):2661-2677. doi: 10.1111/bph.14689. Epub 2019 Jun 26. Br J Pharmacol. 2019. PMID: 30981211 Free PMC article.
-
Pleiotropic Effects of the NSAID Fenamates on Chloride Channels: Opportunity for Ion Channelopathies?Pharmacol Res Perspect. 2025 Aug;13(4):e70144. doi: 10.1002/prp2.70144. Pharmacol Res Perspect. 2025. PMID: 40568930 Free PMC article. Review.
-
Subunit-Specific Photocontrol of Glycine Receptors by Azobenzene-Nitrazepam Photoswitcher.eNeuro. 2021 Jan 21;8(1):ENEURO.0294-20.2020. doi: 10.1523/ENEURO.0294-20.2020. Print 2021 Jan-Feb. eNeuro. 2021. PMID: 33298457 Free PMC article.
References
LinkOut - more resources
Full Text Sources

