Tacrine(10)-Hupyridone Prevents Post-operative Cognitive Dysfunction via the Activation of BDNF Pathway and the Inhibition of AChE in Aged Mice
- PMID: 30483056
- PMCID: PMC6243707
- DOI: 10.3389/fncel.2018.00396
Tacrine(10)-Hupyridone Prevents Post-operative Cognitive Dysfunction via the Activation of BDNF Pathway and the Inhibition of AChE in Aged Mice
Abstract
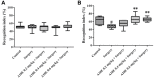
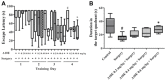
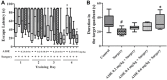
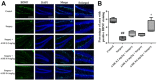
Post-operative cognitive dysfunction (POCD) could cause short-term or long-term cognitive disruption lasting weeks or months after anesthesia and surgery in elderly. However, no effective treatment of POCD is currently available. Previous studies indicated that the enhancement of brain-derived neurotrophic factor (BDNF) expression, and the elevation the cholinergic system, might be effective to prevent POCD. In this study, we have discovered that tacrine(10)-hupyridone (A10E), a novel acetylcholinesterase (AChE) inhibitor derived from tacrine and huperzine A, could prevent surgery-induced short-term and long-term impairments of recognition and spatial cognition, as evidenced by the novel object recognition test and Morris water maze (MWM) tests, in aged mice. Moreover, A10E significantly increased the expression of BDNF and activated the downstream Akt and extracellular regulated kinase (ERK) signaling in the surgery-treated mice. Furthermore, A10E substantially enhanced choline acetyltransferase (ChAT)-positive area and decreased AChE activity, in the hippocampus regions of surgery-treated mice, indicating that A10E could prevent surgery-induced dysfunction of cholinergic system, possibly via increasing the synthesis of acetylcholine and the inhibition of AChE. In conclusion, our results suggested that A10E might prevent POCD via the activation of BDNF pathway and the inhibition of AChE, concurrently, in aged mice. These findings also provided a support that A10E might be developed as a potential drug lead for POCD.
Keywords: acetylcholinesterase; brain-derived neurotrophic factor; choline acetyltransferase; post-operative cognitive dysfunction; tacrine(10)-hupyridone.
Figures





Similar articles
-
Dimeric Tacrine(10)-hupyridone as a Multitarget-Directed Ligand To Treat Alzheimer's Disease.ACS Chem Neurosci. 2021 Jul 7;12(13):2462-2477. doi: 10.1021/acschemneuro.1c00182. Epub 2021 Jun 22. ACS Chem Neurosci. 2021. PMID: 34156230
-
Tacrine(10)-hupyridone, a dual-binding acetylcholinesterase inhibitor, potently attenuates scopolamine-induced impairments of cognition in mice.Metab Brain Dis. 2018 Aug;33(4):1131-1139. doi: 10.1007/s11011-018-0221-7. Epub 2018 Mar 21. Metab Brain Dis. 2018. PMID: 29564727
-
Curcumin attenuates surgery-induced cognitive dysfunction in aged mice.Metab Brain Dis. 2017 Jun;32(3):789-798. doi: 10.1007/s11011-017-9970-y. Epub 2017 Feb 21. Metab Brain Dis. 2017. PMID: 28224377
-
East meets West in the search for Alzheimer's therapeutics - novel dimeric inhibitors from tacrine and huperzine A.Curr Alzheimer Res. 2007 Sep;4(4):386-96. doi: 10.2174/156720507781788918. Curr Alzheimer Res. 2007. PMID: 17908041 Review.
-
Genetic Association of Phosphodiesterases With Human Cognitive Performance.Front Mol Neurosci. 2019 Feb 8;12:22. doi: 10.3389/fnmol.2019.00022. eCollection 2019. Front Mol Neurosci. 2019. PMID: 30800055 Free PMC article. Review.
Cited by
-
Promising tacrine/huperzine A-based dimeric acetylcholinesterase inhibitors for neurodegenerative disorders: From relieving symptoms to modifying diseases through multitarget.J Neurochem. 2021 Sep;158(6):1381-1393. doi: 10.1111/jnc.15379. Epub 2021 Jul 5. J Neurochem. 2021. PMID: 33930191 Free PMC article. Review.
-
Inhibition of acetylcholinesterase activity and β-amyloid oligomer formation by 6-bromotryptamine A, a multi-target anti-Alzheimer's molecule.Oncol Lett. 2020 Feb;19(2):1593-1601. doi: 10.3892/ol.2019.11226. Epub 2019 Dec 18. Oncol Lett. 2020. PMID: 31966085 Free PMC article.
-
Clemastine Ameliorates Perioperative Neurocognitive Disorder in Aged Mice Caused by Anesthesia and Surgery.Front Pharmacol. 2021 Aug 23;12:738590. doi: 10.3389/fphar.2021.738590. eCollection 2021. Front Pharmacol. 2021. PMID: 34497527 Free PMC article.
-
Association between BDNF rs6265 polymorphisms and postoperative cognitive dysfunction in Chinese Han Population.Brain Behav. 2020 Oct;10(10):e01800. doi: 10.1002/brb3.1800. Epub 2020 Aug 17. Brain Behav. 2020. PMID: 33405375 Free PMC article.
-
Update on the Mechanism and Treatment of Sevoflurane-Induced Postoperative Cognitive Dysfunction.Front Aging Neurosci. 2021 Jul 8;13:702231. doi: 10.3389/fnagi.2021.702231. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34305576 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

