Cell-Biological Requirements for the Generation of Dentate Gyrus Granule Neurons
- PMID: 30483057
- PMCID: PMC6240695
- DOI: 10.3389/fncel.2018.00402
Cell-Biological Requirements for the Generation of Dentate Gyrus Granule Neurons
Abstract
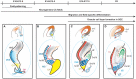
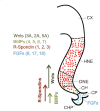
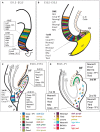
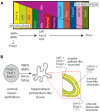
The dentate gyrus (DG) receives highly processed information from the associative cortices functionally integrated in the trisynaptic hippocampal circuit, which contributes to the formation of new episodic memories and the spontaneous exploration of novel environments. Remarkably, the DG is the only brain region currently known to have high rates of neurogenesis in adults (Andersen et al., 1966, 1971). The DG is involved in several neurodegenerative disorders, including clinical dementia, schizophrenia, depression, bipolar disorder and temporal lobe epilepsy. The principal neurons of the DG are the granule cells. DG granule cells generated in culture would be an ideal model to investigate their normal development and the causes of the pathologies in which they are involved and as well as possible therapies. Essential to establish such in vitro models is the precise definition of the most important cell-biological requirements for the differentiation of DG granule cells. This requires a deeper understanding of the precise molecular and functional attributes of the DG granule cells in vivo as well as the DG cells derived in vitro. In this review we outline the neuroanatomical, molecular and cell-biological components of the granule cell differentiation pathway, including some growth- and transcription factors essential for their development. We summarize the functional characteristics of DG granule neurons, including the electrophysiological features of immature and mature granule cells and the axonal pathfinding characteristics of DG neurons. Additionally, we discuss landmark studies on the generation of dorsal telencephalic precursors from pluripotent stem cells (PSCs) as well as DG neuron differentiation in culture. Finally, we provide an outlook and comment critical aspects.
Keywords: dentate gyrus; granule cells; in vitro; induced pluripotent stem cells (iPSC); requirements.
Figures
Similar articles
-
How to make a hippocampal dentate gyrus granule neuron.Development. 2014 Jun;141(12):2366-75. doi: 10.1242/dev.096776. Development. 2014. PMID: 24917496 Review.
-
DNA Methyltransferase 1 Is Indispensable for Development of the Hippocampal Dentate Gyrus.J Neurosci. 2016 Jun 1;36(22):6050-68. doi: 10.1523/JNEUROSCI.0512-16.2016. J Neurosci. 2016. PMID: 27251626 Free PMC article.
-
Prox1 postmitotically defines dentate gyrus cells by specifying granule cell identity over CA3 pyramidal cell fate in the hippocampus.Development. 2012 Aug;139(16):3051-62. doi: 10.1242/dev.080002. Epub 2012 Jul 12. Development. 2012. PMID: 22791897
-
Dentate Gyrus Mossy Cells Share a Role in Pattern Separation with Dentate Granule Cells and Proximal CA3 Pyramidal Cells.J Neurosci. 2019 Nov 27;39(48):9570-9584. doi: 10.1523/JNEUROSCI.0940-19.2019. Epub 2019 Oct 22. J Neurosci. 2019. PMID: 31641051 Free PMC article.
-
Adult neurogenesis in the intact and epileptic dentate gyrus.Prog Brain Res. 2007;163:529-40. doi: 10.1016/S0079-6123(07)63028-3. Prog Brain Res. 2007. PMID: 17765736 Review.
Cited by
-
Dendritic morphology and inhibitory regulation distinguish dentate semilunar granule cells from granule cells through distinct stages of postnatal development.Brain Struct Funct. 2020 Dec;225(9):2841-2855. doi: 10.1007/s00429-020-02162-y. Epub 2020 Oct 29. Brain Struct Funct. 2020. PMID: 33124674 Free PMC article.
-
Neurogenesis From Embryo to Adult - Lessons From Flies and Mice.Front Cell Dev Biol. 2020 Jun 30;8:533. doi: 10.3389/fcell.2020.00533. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32695783 Free PMC article. Review.
-
Repetitive transcranial magnetic stimulation increases neurological function and endogenous neural stem cell migration via the SDF-1α/CXCR4 axis after cerebral infarction in rats.Exp Ther Med. 2021 Sep;22(3):1037. doi: 10.3892/etm.2021.10469. Epub 2021 Jul 19. Exp Ther Med. 2021. PMID: 34373723 Free PMC article.
-
Early postnatal defects in neurogenesis in the 3xTg mouse model of Alzheimer's disease.Cell Death Dis. 2023 Feb 18;14(2):138. doi: 10.1038/s41419-023-05650-1. Cell Death Dis. 2023. PMID: 36801910 Free PMC article.
-
Deconstructing Sox2 Function in Brain Development and Disease.Cells. 2022 May 10;11(10):1604. doi: 10.3390/cells11101604. Cells. 2022. PMID: 35626641 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

