Lowering Etoposide Doses Shifts Cell Demise From Caspase-Dependent to Differentiation and Caspase-3-Independent Apoptosis via DNA Damage Response, Inducing AML Culture Extinction
- PMID: 30483138
- PMCID: PMC6243040
- DOI: 10.3389/fphar.2018.01307
Lowering Etoposide Doses Shifts Cell Demise From Caspase-Dependent to Differentiation and Caspase-3-Independent Apoptosis via DNA Damage Response, Inducing AML Culture Extinction
Abstract
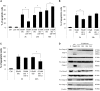
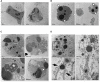
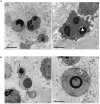
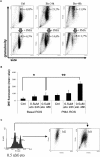
Cytotoxic chemotherapy, still the most widely adopted anticancer treatment, aims at eliminating cancer cells inducing apoptosis with DNA damaging agents, exploiting the differential replication rate of cancer vs. normal cells; efficiency is evaluated in terms of extent of induced apoptosis, which depends on the individual cell sensitivity to a given drug, and on the dose. In this in vitro study, we report that the concentration of etoposide, a topoisomerase II poison widely used in clinics, determines both the kinetics of cell death, and the type of apoptosis induced. We observed that on a set of myeloid leukemia cell lines, etoposide at high (50 uM) dose promoted a rapid caspase-3-mediated apoptosis, whereas at low (0.5 uM) dose, it induced morphological and functional granulocytic differentiation and caspase-2-dependent, but caspase-3-independent, cell death, displaying features consistent with apoptosis. Both differentiation and caspase-2- (but not 3)-mediated apoptosis were contrasted by caffeine, a well-known inhibitor of the cellular DNA damage response (DDR), which maintained cell viability and cycling, indicating that the effects of low etoposide dose are not the immediate consequence of damage, but the result of a signaling pathway. DDR may be thus the mediator responsible for translating a mere dosage-effect into different signal transduction pathways, highlighting a strategic action in regulating timing and mode of cell death according to the severity of induced damage. The evidence of different molecular pathways induced by high vs. low drug doses may possibly contribute to explain the different effects of cytotoxic vs. metronomic therapy, the latter achieving durable clinical responses by treating cancer patients with stable, low doses of otherwise canonical cytotoxic drugs; intriguingly caspase-3, a major promoter of wounded tissue regeneration, is also a key factor of post-therapy cancer repopulation. All this suggests that cancer control in response to cytotoxic drugs arises from complex reprogramming mechanisms in tumor tissue, recently described as anakoinosis.
Keywords: AML; apoptosis; caspase-2; caspase-3; differentiation; metronomic chemotherapy.
Figures

References
-
- Agarwal C., Tyagi A., Agarwal R. (2006). Gallic acid causes inactivating phosphorylation of cdc25A/cdc25C-cdc2 via ATM-Chk2 activation, leading to cell cycle arrest, and induces apoptosis in human prostate carcinoma DU145 cells. Mol. Cancer Ther. 5 3294–3302.10.1158/1535-7163.mct-06-0483 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

