How to Contribute to the Progress of Neuroendocrinology: Discovery of GnIH and Progress of GnIH Research
- PMID: 30483217
- PMCID: PMC6241250
- DOI: 10.3389/fendo.2018.00662
How to Contribute to the Progress of Neuroendocrinology: Discovery of GnIH and Progress of GnIH Research
Abstract
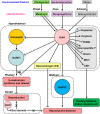
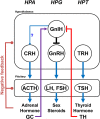
It is essential to discover novel neuropeptides that regulate the functions of pituitary, brain and peripheral secretory glands for the progress of neuroendocrinology. Gonadotropin-releasing hormone (GnRH), a hypothalamic neuropeptide stimulating gonadotropin release was isolated and its structure was determined by Schally's and Guillemin's groups at the beginning of the 1970s. It was subsequently shown that GnRH is highly conserved among vertebrates. GnRH was assumed the sole hypothalamic neuropeptide that regulates gonadotropin release in vertebrates based on extensive studies of GnRH over the following three decades. However, in 2000, Tsutsui's group isolated and determined the structure of a novel hypothalamic neuropeptide, which inhibits gonadotropin release, in quail, an avian species, and named it gonadotropin-inhibitory hormone (GnIH). Following studies by Tsutsui's group demonstrated that GnIH is highly conserved among vertebrates, from humans to agnathans, and acts as a key neuropeptide inhibiting reproduction. Intensive research on GnIH demonstrated that GnIH inhibits gonadotropin synthesis and release by acting on gonadotropes and GnRH neurons via GPR147 in birds and mammals. Fish GnIH also regulates gonadotropin release according to its reproductive condition, indicating the conserved role of GnIH in the regulation of the hypothalamic-pituitary-gonadal (HPG) axis in vertebrates. Therefore, we can now say that GnRH is not the only hypothalamic neuropeptide controlling vertebrate reproduction. In addition, recent studies by Tsutsui's group demonstrated that GnIH acts in the brain to regulate behaviors, including reproductive behavior. The 18 years of GnIH research with leading laboratories in the world have significantly advanced our knowledge of the neuroendocrine control mechanism of reproductive physiology and behavior as well as interactions of the HPG, hypothalamic-pituitary-adrenal and hypothalamic-pituitary-thyroid axes. This review describes how GnIH was discovered and GnIH research progressed in this new research era of reproductive neuroendocrinology.
Keywords: glucocorticoid; gonadotropin-inhibitory hormone (GnIH); gonadotropin-releasing hormone (GnRH); gonadotropins; melatonin; norepinephrine; reproduction; thyroid hormone.
Figures

Similar articles
-
Discovery of gonadotropin-inhibitory hormone (GnIH), progress in GnIH research on reproductive physiology and behavior and perspective of GnIH research on neuroendocrine regulation of reproduction.Mol Cell Endocrinol. 2020 Aug 20;514:110914. doi: 10.1016/j.mce.2020.110914. Epub 2020 Jun 12. Mol Cell Endocrinol. 2020. PMID: 32535039 Review.
-
Gonadotropin-inhibitory hormone (GnIH): A new key neurohormone controlling reproductive physiology and behavior.Front Neuroendocrinol. 2021 Apr;61:100900. doi: 10.1016/j.yfrne.2021.100900. Epub 2021 Jan 12. Front Neuroendocrinol. 2021. PMID: 33450199 Review.
-
Gonadotropin-inhibitory hormone (GnIH) and its control of central and peripheral reproductive function.Front Neuroendocrinol. 2010 Jul;31(3):284-95. doi: 10.1016/j.yfrne.2010.03.001. Epub 2010 Mar 6. Front Neuroendocrinol. 2010. PMID: 20211640 Review.
-
Gonadotropin-inhibitory hormone (GnIH): discovery, progress and prospect.Gen Comp Endocrinol. 2012 Jul 1;177(3):305-14. doi: 10.1016/j.ygcen.2012.02.013. Epub 2012 Feb 26. Gen Comp Endocrinol. 2012. PMID: 22391238 Free PMC article. Review.
-
Advancing reproductive neuroendocrinology through research on the regulation of GnIH and on its diverse actions on reproductive physiology and behavior.Front Neuroendocrinol. 2022 Jan;64:100955. doi: 10.1016/j.yfrne.2021.100955. Epub 2021 Nov 9. Front Neuroendocrinol. 2022. PMID: 34767778 Review.
Cited by
-
Food deprivation differentially modulates gene expression of LPXRFa and kisspeptin systems in the brain-pituitary axis of half-smooth tongue sole (Cynoglossus semilaevis).Front Endocrinol (Lausanne). 2023 Mar 24;14:1099832. doi: 10.3389/fendo.2023.1099832. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37033260 Free PMC article.
-
Brain RFamide Neuropeptides in Stress-Related Psychopathologies.Cells. 2024 Jun 25;13(13):1097. doi: 10.3390/cells13131097. Cells. 2024. PMID: 38994950 Free PMC article. Review.
-
No evidence for an association between Clock gene allelic variation and migration timing in a long-distance migratory shorebird (Limosa lapponica baueri).Oecologia. 2019 Dec;191(4):843-859. doi: 10.1007/s00442-019-04524-8. Epub 2019 Oct 28. Oecologia. 2019. PMID: 31659437
-
Fluctuating and Stable High Temperatures Differentially Affect Reproductive Endocrinology of Female Pupfish.Integr Org Biol. 2024 Feb 1;6(1):obae003. doi: 10.1093/iob/obae003. eCollection 2024. Integr Org Biol. 2024. PMID: 38464886 Free PMC article.
-
Molecular Mechanisms of Gonadotropin-Inhibitory Hormone (GnIH) Actions in Target Cells and Regulation of GnIH Expression.Front Endocrinol (Lausanne). 2019 Feb 25;10:110. doi: 10.3389/fendo.2019.00110. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 30858828 Free PMC article. Review.
References
-
- Livermore AH, Du Vigneaud V. Preparation of high potency oxytocic material by the use of counter-current distribution. J Biol Chem. (1949) 180:365–73. - PubMed
-
- Turner RA, Pierce JG, Du Vigneaud V. The purification and the amino acid content of vasopressin preparations. J Biol Chem. (1951) 191:21–8. - PubMed
-
- Harris GW. Neuronal control of the pituitary gland. Physiol Rev. (1948) 28:139–79. - PubMed
-
- Burgus R, Dunn TF, Desiderio D, Guillemin R. Molecular structure of the hypothalamic hypophysiotropic TRF factor of ovine origin: mass spectrometry demonstration of the PCA-His-Pro-NH2 sequence. C R Acad Sci (Paris) (1969) 269:1870–3. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

