In vitro and in vivo Effects of Lactate on Metabolism and Cytokine Production of Human Primary PBMCs and Monocytes
- PMID: 30483253
- PMCID: PMC6240653
- DOI: 10.3389/fimmu.2018.02564
In vitro and in vivo Effects of Lactate on Metabolism and Cytokine Production of Human Primary PBMCs and Monocytes
Abstract
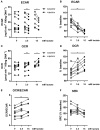
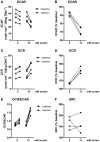
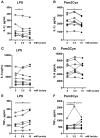
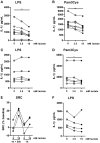
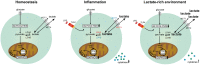
Lactate, the end product of anaerobic glycolysis, is produced in high amounts by innate immune cells during inflammatory activation. Although immunomodulating effects of lactate have been reported, evidence from human studies is scarce. Here we show that expression of genes involved in lactate metabolism and transport is modulated in human immune cells during infection and upon inflammatory activation with TLR ligands in vitro, indicating an important role for lactate metabolism in inflammation. Extracellular lactate induces metabolic reprogramming in innate immune cells, as evidenced by reduced glycolytic and increased oxidative rates of monocytes immediately after exposure to lactate. A short-term infusion of lactate in humans in vivo increased ex vivo glucose consumption of PBMCs, but effects on metabolic rates and cytokine production were limited. Interestingly, long-term treatment with lactate ex vivo, reflecting pathophysiological conditions in local microenvironments such as tumor or adipose tissue, significantly modulated cytokine production with predominantly anti-inflammatory effects. We found time- and stimuli-dependent effects of extracellular lactate on cytokine production, further emphasizing the complex interplay between metabolism and immune cell function. Together, our findings reveal lactate as a modulator of immune cell metabolism which translates to reduced inflammation and may ultimately function as a negative feedback signal to prevent excessive inflammatory responses.
Keywords: cytokines; glycolysis; immunometabolism; innate immune cells; lactate; monocytes.
Figures






Similar articles
-
Vitamin D [1,25(OH)2D3] Differentially Regulates Human Innate Cytokine Responses to Bacterial versus Viral Pattern Recognition Receptor Stimuli.J Immunol. 2016 Apr 1;196(7):2965-72. doi: 10.4049/jimmunol.1500460. Epub 2016 Feb 19. J Immunol. 2016. PMID: 26895836
-
Rewiring of glucose metabolism defines trained immunity induced by oxidized low-density lipoprotein.J Mol Med (Berl). 2020 Jun;98(6):819-831. doi: 10.1007/s00109-020-01915-w. Epub 2020 Apr 30. J Mol Med (Berl). 2020. PMID: 32350546 Free PMC article.
-
Lactic acid and acidification inhibit TNF secretion and glycolysis of human monocytes.J Immunol. 2010 Feb 1;184(3):1200-9. doi: 10.4049/jimmunol.0902584. Epub 2009 Dec 21. J Immunol. 2010. PMID: 20026743
-
Lactate and Immunosuppression in Sepsis.Shock. 2018 Feb;49(2):120-125. doi: 10.1097/SHK.0000000000000958. Shock. 2018. PMID: 28767543 Free PMC article. Review.
-
The Immune Consequences of Lactate in the Tumor Microenvironment.Adv Exp Med Biol. 2020;1259:113-124. doi: 10.1007/978-3-030-43093-1_7. Adv Exp Med Biol. 2020. PMID: 32578174 Review.
Cited by
-
Glycolytic activity in human immune cells: inter-individual variation and functional implications during health and diabetes.Immunometabolism (Cobham). 2022 Nov 1;4(4):e00008. doi: 10.1097/IN9.0000000000000008. eCollection 2022 Oct. Immunometabolism (Cobham). 2022. PMID: 36337734 Free PMC article.
-
Lactate produced by alveolar type II cells suppresses inflammatory alveolar macrophages in acute lung injury.FASEB J. 2023 Dec;37(12):e23316. doi: 10.1096/fj.202301722R. FASEB J. 2023. PMID: 37983890 Free PMC article.
-
Gut microbiota differences between psoriatic arthritis and other undifferentiated arthritis: A pilot study.Medicine (Baltimore). 2022 Jul 15;101(28):e29870. doi: 10.1097/MD.0000000000029870. Medicine (Baltimore). 2022. PMID: 35839060 Free PMC article.
-
The Microbiome as a Key Regulator of Female Genital Tract Barrier Function.Front Cell Infect Microbiol. 2021 Dec 17;11:790627. doi: 10.3389/fcimb.2021.790627. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34976864 Free PMC article. Review.
-
Lactate and lactylation: emerging roles in autoimmune diseases and metabolic reprogramming.Front Immunol. 2025 Jun 27;16:1589853. doi: 10.3389/fimmu.2025.1589853. eCollection 2025. Front Immunol. 2025. PMID: 40655145 Free PMC article. Review.
References
-
- DiGirolamo M, Newby FD, Lovejoy J. Lactate production in adipose tissue: a regulated function with extra-adipose implications. FASEB J. (1992) 6:2405–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

