Coffee Somatic Embryogenesis: How Did Research, Experience Gained and Innovations Promote the Commercial Propagation of Elite Clones From the Two Cultivated Species?
- PMID: 30483287
- PMCID: PMC6240679
- DOI: 10.3389/fpls.2018.01630
Coffee Somatic Embryogenesis: How Did Research, Experience Gained and Innovations Promote the Commercial Propagation of Elite Clones From the Two Cultivated Species?
Abstract
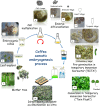
Since the 1990s, somatic embryogenesis (SE) has enabled the propagation of selected varieties, Arabica F1 hybrid and Robusta clones, originating from the two cultivated coffee species, Coffea arabica and Coffea canephora, respectively. This paper shows how mostly empirical research has led to successful industrial transfers launched in the 2000s in Latin America, Africa, and Asia. Coffee SE can be considered as a model for other woody perennial crops for the following reasons: (i) a high biological efficiency has been demonstrated for propagated varieties at all developmental stages, and (ii) somaclonal variation is understood and mastered thanks to intensive research combining molecular markers and field observations. Coffee SE is also a useful model given the strong economic constraints that are specific to this species. In brief, SE faced four difficulties: (i) the high cost of SE derived plants compared to the cost of seedlings of conventional varieties, (ii) the logistic problems involved in reaching small-scale coffee growers, (iii) the need for certification, and (iv) the lack of solvency among small-scale producers. Nursery activities were professionalized by introducing varietal certification, quality control with regard to horticultural problems and somaclonal variation, and sanitary control for Xylella fastidiosa. In addition, different technology transfers were made to ensure worldwide dissemination of improved F1 Arabica hybrids and Robusta clones. Innovations have been decisive for successful scaling-up and reduction of production costs, such as the development of temporary immersion bioreactors for the mass production of pre-germinated embryos, their direct sowing on horticultural soil, and the propagation of rejuvenated SE plants by rooted mini-cuttings. Today, SE is a powerful tool that is widely used in coffee for biotechnological applications including propagation and genetic transformation. Basic research has recently started taking advantage of optimized SE protocols. Based on -omics methodologies, research aims to decipher the molecular events involved in the key developmental switches of coffee SE. In parallel, a high-throughput screening of active molecules on SE appears to be a promising tool to speed-up the optimization of SE protocols.
Keywords: bioreactors; cuttings; innovation; somaclonal variation; technology transfer.
Figures










References
-
- Arroyo-Herrera A., Ku Gonzalez A., Canche Moo R., Quiroz-Figueroa F. R., Loyola-Vargas V. M., Rodriguez-Zapata L. C., et al. (2008). Expression of WUSCHEL in Coffea canephora causes ectopic morphogenesis and increases somatic embryogenesis. Plant Cell Tissue Organ Cult. 94 171–180. 10.1007/s11240-008-9401-1 - DOI
Publication types
LinkOut - more resources
Full Text Sources

