Relational concurrency, stages of infection, and the evolution of HIV set point viral load
- PMID: 30483403
- PMCID: PMC6249390
- DOI: 10.1093/ve/vey032
Relational concurrency, stages of infection, and the evolution of HIV set point viral load
Abstract
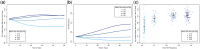
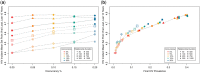
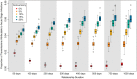
HIV viral load (VL) predicts both transmission potential and rate of disease progression. For reasons that are still not fully understood, the set point viral load (SPVL) established after acute infection varies across individuals and populations. Previous studies have suggested that population mean SPVL (MSPVL) has evolved near an optimum that reflects a trade-off between transmissibility and host survival. Sexual network structures affect rates of potential exposure during different within-host phases of infection marked by different transmission probabilities, and thus affect the number and timing of transmission events. These structures include relational concurrency, which has been argued to explain key differences in HIV burden across populations. We hypothesize that concurrency will alter the fitness landscape for SPVL in ways that differ from other network features whose impacts accrue at other times during infection. To quantitatively test this hypothesis, we developed a dynamic, stochastic, data-driven network model of HIV transmission, and evolution to assess the impact of key sexual network phenomena on MSPVL evolution. Experiments were repeated in sensitivity runs that made different assumptions about transmissibility during acute infection, SPVL heritability, and the functional form of the relationship between VL and transmissibility. For our main transmission model, scenarios yielded MSPVLs ranging from 4.4 to 4.75 log10 copies/ml, covering much of the observed empirical range. MSPVL evolved to be higher in populations with high concurrency and shorter relational durations, with values varying over a clinically significant range. In linear regression analyses on these and other predictors, main effects were significant (P < 0.05), as were interaction terms, indicating that effects are interdependent. We also noted a strong correlation between two key emergent properties measured at the end of the simulations-MSPVL and HIV prevalence-most clearly for phenomena that affect transmission networks early in infection. Controlling for prevalence, high concurrency yielded higher MSPVL than other network phenomena. Interestingly, we observed lower prevalence in runs in which SPVL heritability was zero, indicating the potential for viral evolution to exacerbate disease burden over time. Future efforts to understand empirical variation in MSPVL should consider local HIV burden and basic sexual behavioral and network structure.
Keywords: HIV-1; relational concurrency; set point viral load; sexual networks; viral evolution.
Figures
Similar articles
-
Test-and-treat coverage and HIV virulence evolution among men who have sex with men.Virus Evol. 2021 Feb 10;7(1):veab011. doi: 10.1093/ve/veab011. eCollection 2021 Jan. Virus Evol. 2021. PMID: 33633867 Free PMC article.
-
Why does age at HIV infection correlate with set point viral load? An evolutionary hypothesis.Epidemics. 2022 Dec;41:100629. doi: 10.1016/j.epidem.2022.100629. Epub 2022 Sep 21. Epidemics. 2022. PMID: 36162386 Free PMC article.
-
Virus-induced target cell activation reconciles set-point viral load heritability and within-host evolution.Epidemics. 2013 Dec;5(4):174-80. doi: 10.1016/j.epidem.2013.09.002. Epub 2013 Sep 23. Epidemics. 2013. PMID: 24267873
-
Potential Pitfalls in Estimating Viral Load Heritability.Trends Microbiol. 2016 Sep;24(9):687-698. doi: 10.1016/j.tim.2016.04.008. Epub 2016 May 12. Trends Microbiol. 2016. PMID: 27185643 Review.
-
Interaction of the Host and Viral Genome and Their Influence on HIV Disease.Front Genet. 2019 Jan 23;9:720. doi: 10.3389/fgene.2018.00720. eCollection 2018. Front Genet. 2019. PMID: 30728828 Free PMC article. Review.
Cited by
-
Evolution of HIV virulence in response to disease-modifying vaccines: A modeling study.Vaccine. 2023 Oct 13;41(43):6461-6469. doi: 10.1016/j.vaccine.2023.08.071. Epub 2023 Sep 14. Vaccine. 2023. PMID: 37714749 Free PMC article.
-
Comparison of two simulators for individual based models in HIV epidemiology in a population with HSV 2 in Yaoundé (Cameroon).Sci Rep. 2021 Jul 19;11(1):14696. doi: 10.1038/s41598-021-94289-z. Sci Rep. 2021. PMID: 34282252 Free PMC article.
-
Test-and-treat coverage and HIV virulence evolution among men who have sex with men.Virus Evol. 2021 Feb 10;7(1):veab011. doi: 10.1093/ve/veab011. eCollection 2021 Jan. Virus Evol. 2021. PMID: 33633867 Free PMC article.
-
Large benefits to youth-focused HIV treatment-as-prevention efforts in generalized heterosexual populations: An agent-based simulation model.PLoS Comput Biol. 2019 Dec 17;15(12):e1007561. doi: 10.1371/journal.pcbi.1007561. eCollection 2019 Dec. PLoS Comput Biol. 2019. PMID: 31846456 Free PMC article.
-
Why does age at HIV infection correlate with set point viral load? An evolutionary hypothesis.Epidemics. 2022 Dec;41:100629. doi: 10.1016/j.epidem.2022.100629. Epub 2022 Sep 21. Epidemics. 2022. PMID: 36162386 Free PMC article.
References
-
- Alizon S. et al. (2009) ‘Virulence Evolution and the Trade-off Hypothesis: History, Current State of Affairs and the Future’, Journal of Evolutionary Biology, 22: 245–59. - PubMed
-
- Andre J. B., Hochberg M. E. (2005) ‘Virulence Evolution in Emerging Infectious Diseases’, Evolution, 59: 1406–12. - PubMed
-
- Beyrer C. et al. (2010) ‘Bisexual Concurrency, Bisexual Partnerships, and HIV among Southern African Men Who Have Sex with Men’, Sexually Transmitted Infections, 86: 323–7. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

