Role of Metabolism in Hepatic Stellate Cell Activation and Fibrogenesis
- PMID: 30483502
- PMCID: PMC6240744
- DOI: 10.3389/fcell.2018.00150
Role of Metabolism in Hepatic Stellate Cell Activation and Fibrogenesis
Abstract
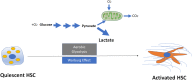
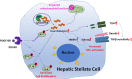
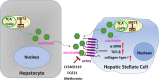
Activation of hepatic stellate cell (HSC) involves the transition from a quiescent to a proliferative, migratory, and fibrogenic phenotype (i.e., myofibroblast), which is characteristic of liver fibrogenesis. Multiple cellular and molecular signals which contribute to HSC activation have been identified. This review specially focuses on the metabolic changes which impact on HSC activation and fibrogenesis.
Keywords: fibroblast; glutaminolysis; glycolysis; liver fibrosis; metabolic.
Figures


Similar articles
-
Cooperation of liver cells in health and disease.Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. doi: 10.1007/978-3-642-56553-3. Adv Anat Embryol Cell Biol. 2001. PMID: 11729749 Review.
-
Molecular interplays in hepatic stellate cells: apoptosis, senescence, and phenotype reversion as cellular connections that modulate liver fibrosis.Cell Biol Int. 2017 Sep;41(9):946-959. doi: 10.1002/cbin.10790. Epub 2017 Jul 20. Cell Biol Int. 2017. PMID: 28498509 Review.
-
Overexpression of c-myc in hepatocytes promotes activation of hepatic stellate cells and facilitates the onset of liver fibrosis.Biochim Biophys Acta. 2013 Oct;1832(10):1765-75. doi: 10.1016/j.bbadis.2013.06.001. Epub 2013 Jun 12. Biochim Biophys Acta. 2013. PMID: 23770341
-
Therapeutic effects of serum extracellular vesicles in liver fibrosis.J Extracell Vesicles. 2018 Apr 17;7(1):1461505. doi: 10.1080/20013078.2018.1461505. eCollection 2018. J Extracell Vesicles. 2018. PMID: 29696080 Free PMC article.
-
SIRT1 antagonizes liver fibrosis by blocking hepatic stellate cell activation in mice.FASEB J. 2018 Jan;32(1):500-511. doi: 10.1096/fj.201700612R. Epub 2017 Sep 26. FASEB J. 2018. PMID: 28970250
Cited by
-
A novel minimally invasive OFM technique with orthotopic transplantation of hUC-MSCs and in vivo monitoring of liver metabolic microenvironment in liver fibrosis treatment.Stem Cell Res Ther. 2021 Oct 9;12(1):534. doi: 10.1186/s13287-021-02599-w. Stem Cell Res Ther. 2021. PMID: 34627378 Free PMC article.
-
Fibrogenic Pathways in Metabolic Dysfunction Associated Fatty Liver Disease (MAFLD).Int J Mol Sci. 2022 Jun 23;23(13):6996. doi: 10.3390/ijms23136996. Int J Mol Sci. 2022. PMID: 35805998 Free PMC article. Review.
-
Mitofusin-2 Restrains Hepatic Stellate Cells' Proliferation via PI3K/Akt Signaling Pathway and Inhibits Liver Fibrosis in Rats.J Healthc Eng. 2022 Jan 17;2022:6731335. doi: 10.1155/2022/6731335. eCollection 2022. J Healthc Eng. 2022. PMID: 35083025 Free PMC article.
-
Gut microbiota mediated molecular events and therapy in liver diseases.World J Gastroenterol. 2020 Dec 28;26(48):7603-7618. doi: 10.3748/wjg.v26.i48.7603. World J Gastroenterol. 2020. PMID: 33505139 Free PMC article. Review.
-
Treatment of liver fibrosis: Past, current, and future.World J Hepatol. 2023 Jun 27;15(6):755-774. doi: 10.4254/wjh.v15.i6.755. World J Hepatol. 2023. PMID: 37397931 Free PMC article. Review.
References
-
- Ajat M., Molenaar M., Brouwers J. F. H. M., Vaandrager A. B., Houweling M., Helms J. B. (2017). Hepatic stellate cells retain the capacity to synthesize retinyl esters and to store neutral lipids in small lipid droplets in the absence of LRAT. Biochim. Biophys. Acta 1862 176–187. 10.1016/j.bbalip.2016.10.013 - DOI - PubMed
LinkOut - more resources
Full Text Sources

