Conformational Change Induced by Putidaredoxin Binding to Ferrous CO-ligated Cytochrome P450cam Characterized by 2D IR Spectroscopy
- PMID: 30483514
- PMCID: PMC6243089
- DOI: 10.3389/fmolb.2018.00094
Conformational Change Induced by Putidaredoxin Binding to Ferrous CO-ligated Cytochrome P450cam Characterized by 2D IR Spectroscopy
Abstract
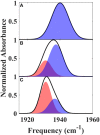
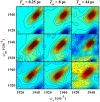
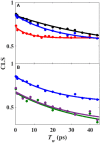
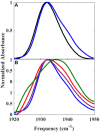
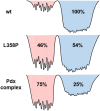
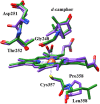
The importance of conformational dynamics to protein function is now well-appreciated. An outstanding question is whether they are involved in the effector role played by putidaredoxin (Pdx) in its reduction of the O2 complex of cytochrome P450cam (P450cam), an archetypical member of the cytochrome P450 superfamily. Recent studies have reported that binding of Pdx induces a conformational change from a closed to an open state of ferric P450cam, but a similar conformational change does not appear to occur for the ferrous, CO-ligated enzyme. To better understand the effector role of Pdx when binding the ferrous, CO-ligated P450cam, we applied 2D IR spectroscopy to compare the conformations and dynamics of the wild-type (wt) enzyme in the absence and presence of Pdx, as well as of L358P P450cam (L358P), which has served as a putative model for the Pdx complex. The CO vibrations of the Pdx complex and L358P report population of two conformational states in which the CO experiences distinct environments. The dynamics among the CO frequencies indicate that the energy landscape of substates within one conformation are reflective of the closed state of P450cam, and for the other conformation, differ from the free wt enzyme, but are equivalent between the Pdx complex and L358P. The two states co-populated by the Pdx complex are postulated to reflect a loosely bound encounter complex and a more tightly bound state, as is commonly observed for the dynamic complexes of redox partners. Significantly, this study shows that the binding of Pdx to ferrous, CO-ligated P450cam does perturb the conformational ensemble in a way that might underlie the effector role of Pdx.
Keywords: 2D IR spectroscopy; cytochrome P450; energy landscape; infrared spectroscopy; protein dynamics; putidaredoxin.
Figures
Similar articles
-
Effector Roles of Putidaredoxin on Cytochrome P450cam Conformational States.J Am Chem Soc. 2016 Aug 17;138(32):10163-72. doi: 10.1021/jacs.6b04110. Epub 2016 Aug 5. J Am Chem Soc. 2016. PMID: 27452076
-
L358P mutation on cytochrome P450cam simulates structural changes upon putidaredoxin binding: the structural changes trigger electron transfer to oxy-P450cam from electron donors.J Biol Chem. 2004 Oct 8;279(41):42836-43. doi: 10.1074/jbc.M404216200. Epub 2004 Jul 21. J Biol Chem. 2004. PMID: 15269211
-
Putidaredoxin Binds to the Same Site on Cytochrome P450cam in the Open and Closed Conformation.Biochemistry. 2017 Aug 22;56(33):4371-4378. doi: 10.1021/acs.biochem.7b00564. Epub 2017 Aug 10. Biochemistry. 2017. PMID: 28741929
-
Structural biology of redox partner interactions in P450cam monooxygenase: a fresh look at an old system.Arch Biochem Biophys. 2011 Mar 1;507(1):66-74. doi: 10.1016/j.abb.2010.08.022. Epub 2010 Sep 15. Arch Biochem Biophys. 2011. PMID: 20816746 Free PMC article. Review.
-
Updating the Paradigm: Redox Partner Binding and Conformational Dynamics in Cytochromes P450.Acc Chem Res. 2022 Feb 1;55(3):373-380. doi: 10.1021/acs.accounts.1c00632. Epub 2021 Dec 29. Acc Chem Res. 2022. PMID: 34965086 Free PMC article. Review.
Cited by
-
Active Site Hydrogen Bonding Induced in Cytochrome P450cam by Effector Putidaredoxin.Biochemistry. 2021 Jun 1;60(21):1699-1707. doi: 10.1021/acs.biochem.1c00075. Epub 2021 May 18. Biochemistry. 2021. PMID: 34006086 Free PMC article.
-
Site-Specific 1D and 2D IR Spectroscopy to Characterize the Conformations and Dynamics of Protein Molecular Recognition.J Phys Chem B. 2019 May 2;123(17):3551-3566. doi: 10.1021/acs.jpcb.9b00969. Epub 2019 Mar 21. J Phys Chem B. 2019. PMID: 30848912 Free PMC article.
-
Transparent window 2D IR spectroscopy of proteins.J Chem Phys. 2021 Jul 28;155(4):040903. doi: 10.1063/5.0052628. J Chem Phys. 2021. PMID: 34340394 Free PMC article. Review.
-
Protein Dynamics by Two-Dimensional Infrared Spectroscopy.Annu Rev Anal Chem (Palo Alto Calif). 2021 Jul 27;14(1):299-321. doi: 10.1146/annurev-anchem-091520-091009. Annu Rev Anal Chem (Palo Alto Calif). 2021. PMID: 34314221 Free PMC article.
-
Heterogeneous and Highly Dynamic Interface in Plastocyanin-Cytochrome f Complex Revealed by Site-Specific 2D-IR Spectroscopy.J Phys Chem B. 2019 Mar 7;123(9):2114-2122. doi: 10.1021/acs.jpcb.8b12157. Epub 2019 Feb 21. J Phys Chem B. 2019. PMID: 30742428 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

