Daclatasvir and asunaprevir improves health-related quality of life in Japanese patients infected with hepatitis C virus
- PMID: 30483569
- PMCID: PMC6207024
- DOI: 10.1002/jgh3.12052
Daclatasvir and asunaprevir improves health-related quality of life in Japanese patients infected with hepatitis C virus
Abstract
Aims: Interferon-free direct-acting antiviral agent (DAA) regimens for chronic hepatitis C virus (HCV) patients have improved their health-related quality of life (HRQOL). Currently, there are no published data assessing the impact of DAAs regimens without sofosbuvir on HRQOL. The aim of this study was to investigate the improvement of HRQOL in Japanese HCV patients treated with a protease inhibitor and a nonstructural protein 5A inhibitor.
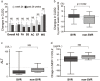
Methods and results: A total of 123 Japanese genotype 1b HCV patients receiving daclatasvir (DCV) and asunaprevir (ASV) for 24 weeks were enrolled. HRQOL was assessed using the Japanese version of the Chronic Liver Disease Questionnaire (CLDQ) at baseline; weeks 4, 12, and 24; and post-24 weeks. Changes in CLDQ scores were calculated by subtracting the CLDQ score at each time point from the baseline value. Improvement in the mean change of the Japanese version of the CLDQ score became statistically significant as early as week 4 after the initiation of treatment (+9.3%; P < 0.0001) and was sustained during and after DCV/ASV treatment. The changes of CLDQ at posttreatment week 24 in patients with sustained virological responses (SVR) were significantly higher than those in patients without SVR (0.4% and -4.1%, respectively; P < 0.05).
Conclusions: This study of DCV/ASV treatment for Japanese HCV patients in a clinical setting demonstrated that HRQOL can improve as early as at the initiation of treatment and can continue during and after treatment, regardless of the classes of DAAs regimens and race. Moreover, SVR are needed to continue HRQOL improvement.
Keywords: asunaprevir; chronic liver disease questionnaire; daclatasvir; health‐related quality of life; hepatitis C virus.
Figures


References
-
- Hepatitis C. guidance: AASLD‐IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015; 62: 932–54. - PubMed
-
- Pawlotsky JM. New hepatitis C therapies: the toolbox, strategies, and challenges. Gastroenterology. 2014; 146: 1176–92. - PubMed
-
- Najafzadeh M, Andersson K, Shrank WH et al Cost‐effectiveness of novel regimens for the treatment of hepatitis C virus. Ann. Intern. Med. 2015; 162: 407–19. - PubMed
-
- Younossi Z, Kallman J, Kincaid J. The effects of HCV infection and management on health‐related quality of life. Hepatology. 2007; 45: 806–16. - PubMed
LinkOut - more resources
Full Text Sources

