A review of vedolizumab and ustekinumab for the treatment of inflammatory bowel diseases
- PMID: 30483594
- PMCID: PMC6207060
- DOI: 10.1002/jgh3.12065
A review of vedolizumab and ustekinumab for the treatment of inflammatory bowel diseases
Abstract
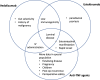
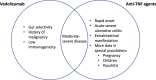
Recent advancement in the understanding of the pathophysiology of inflammatory bowel disease has seen an expansion in therapeutic options. Vedolizumab, a selective α4β7 inhibitor, and ustekinumab, an IL 12/23 p40 inhibitor, have provided the much-awaited out-of-class alternatives for patients who have failed or who are intolerant to anti-Tumor Necrosis Factor (TNF) therapy. However, questions remain as to how we may best use these novel therapeutic agents. We evaluate the evidence available from randomized controlled trials and postmarketing cohort studies and discuss their safety, efficacy, and limitations, in relation to anti-TNF therapy, in optimizing the treatment outcomes.
Keywords: anti‐TNF; inflammatory bowel disease; ustekinumab; vedolizumab.
Figures
References
-
- Targan S, Hanauer S, van Deventer S et al A short‐term study of chimeric monoclonal antibody cA2 to tumor necrosis factor a for Crohn's disease. N. Engl. J. Med. 1997; 337: 1029–35. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources