Oncological hyperthermia: The correct dosing in clinical applications
- PMID: 30483754
- PMCID: PMC6317680
- DOI: 10.3892/ijo.2018.4645
Oncological hyperthermia: The correct dosing in clinical applications
Abstract
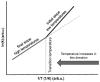
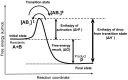
The problem with the application of conventional hyperthermia in oncology is firmly connected to the dose definition, which conventionally uses the concept of the homogeneous (isothermal) temperature of the target. Its imprecise control and complex evaluation is the primary barrier to the extensive clinical applications. The aim of this study was to show the basis of the problems of the misleading dose concept. A clear clarification of the proper dose concept must begin with the description of the limitations of the present doses in conventional hyperthermia applications. The surmounting of the limits the dose of oncologic hyperthermia has to be based on the applicability of the Eyring transition state theory on thermal effects. In order to avoid the countereffects of thermal homeostasis, the use of precise heating on the nanoscale with highly efficient energy delivery is recommended. The nano‑scale heating allows for an energy‑based dose to control the process. The main aspects of the method are the following: i) It is not isothermal (no homogeneous heating); ii) malignant cells are heated selectively; and iii) it employs high heating efficacy, with less energy loss. The applied rigorous thermodynamical considerations show the proper terminology and dose concept of hyperthermia, which is based on the energy‑absorption (such as in the case of ionizing radiation) instead of the temperature‑based ideas. On the whole, according to the present study, the appropriate dose in oncological hyperthermia must use an energy‑based concept, as it is well‑known in all the ionizing radiation therapies. We propose the use of Gy (J/kg) in cases of non‑ionizing radiation (hyperthermia) as well.
Keywords: hyperthermia dose; CEM43Tx; TRISE; specific absorption rate; modulated electro-hyperthermia.
Figures





Similar articles
-
Reduction of peak acoustic pressure and shaping of heated region by use of multifoci sonications in MR-guided high-intensity focused ultrasound mediated mild hyperthermia.Med Phys. 2013 Jan;40(1):013301. doi: 10.1118/1.4769116. Med Phys. 2013. PMID: 23298120 Free PMC article.
-
Quantifying the combined effect of radiation therapy and hyperthermia in terms of equivalent dose distributions.Int J Radiat Oncol Biol Phys. 2014 Mar 1;88(3):739-45. doi: 10.1016/j.ijrobp.2013.11.212. Epub 2014 Jan 8. Int J Radiat Oncol Biol Phys. 2014. PMID: 24411189
-
Quality assurance protocol for superficial and deep hyperthermia systems established by the Hellenic Association of Medical Physicists (HAMP) in cooperation with the Hellenic Society of Oncologic Hyperthemia (HSOH): A study based on European Society for Hyperthermic Oncology (ESHO) quality assurance guidelines.J BUON. 2018 Mar-Apr;23(2):494-499. J BUON. 2018. PMID: 29745098
-
Therapeutic hyperthermia.Handb Clin Neurol. 2018;157:853-868. doi: 10.1016/B978-0-444-64074-1.00053-7. Handb Clin Neurol. 2018. PMID: 30459045 Review.
-
The current and potential role of hyperthermia in radiotherapy.Int J Radiat Oncol Biol Phys. 1989 Mar;16(3):535-49. doi: 10.1016/0360-3016(89)90470-7. Int J Radiat Oncol Biol Phys. 1989. PMID: 2646256 Review.
Cited by
-
Quo Vadis Oncological Hyperthermia (2020)?Front Oncol. 2020 Sep 4;10:1690. doi: 10.3389/fonc.2020.01690. eCollection 2020. Front Oncol. 2020. PMID: 33014841 Free PMC article. Review.
-
Modulated Electrohyperthermia: A New Hope for Cancer Patients.Biomed Res Int. 2020 Nov 13;2020:8814878. doi: 10.1155/2020/8814878. eCollection 2020. Biomed Res Int. 2020. PMID: 33274226 Free PMC article. Review.
-
Bevacizumab Plus FOLFOX-4 Combined With Deep Electro-Hyperthermia as First-line Therapy in Metastatic Colon Cancer: A Pilot Study.Front Oncol. 2020 Nov 3;10:590707. doi: 10.3389/fonc.2020.590707. eCollection 2020. Front Oncol. 2020. PMID: 33224885 Free PMC article.
-
Modulated electro-hyperthermia in stage III and IV pancreatic cancer: Results of an observational study on 158 patients.World J Clin Oncol. 2021 Nov 24;12(11):1064-1071. doi: 10.5306/wjco.v12.i11.1064. World J Clin Oncol. 2021. PMID: 34909400 Free PMC article.
-
Putative Abscopal Effect in Three Patients Treated by Combined Radiotherapy and Modulated Electrohyperthermia.Front Oncol. 2020 Mar 10;10:254. doi: 10.3389/fonc.2020.00254. eCollection 2020. Front Oncol. 2020. PMID: 32211319 Free PMC article.
References
-
- Roussakow S. The History Of Hyperthermia Rise And Decline Hindawi Publishing Corporation; Conference Papers in Medicine 2013; 2013.p. 3428027.
-
- Oncology Encyclopedia MedicineNet Hyperthermia definition, Answers. 2008 http://www.answers.com/topic/hyper-thermia. Accessed May 23, 2018.
-
- Medicine.net Hyperthermia definition. 2008 http://www.medterms.com/script/main/art.asp?articlekey=3848. Accessed May 23, 2018.
MeSH terms
LinkOut - more resources
Full Text Sources

