MYCN is amplified during S phase, and c‑myb is involved in controlling MYCN expression and amplification in MYCN‑amplified neuroblastoma cell lines
- PMID: 30483774
- PMCID: PMC6297758
- DOI: 10.3892/mmr.2018.9686
MYCN is amplified during S phase, and c‑myb is involved in controlling MYCN expression and amplification in MYCN‑amplified neuroblastoma cell lines
Abstract
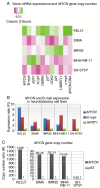
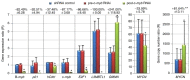
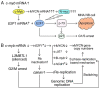
Neuroblastoma derived from primitive sympathetic neural precursors is a common type of solid tumor in infants. MYCN proto‑oncogene bHLH transcription factor (MYCN) amplification and 1p36 deletion are important factors associated with the poor prognosis of neuroblastoma. Expression levels of MYCN and c‑MYB proto‑oncogene transcription factor (c‑myb) decline during the differentiation of neuroblastoma cells; E2F transcription factor 1 (E2F1) activates the MYCN promoter. However, the underlying mechanism of MYCN overexpression and amplification requires further investigation. In the present study, potential c‑Myb target genes, and the effect of c‑myb RNA interference (RNAi) on MYCN expression and amplification were investigated in MYCN‑amplified neuroblastoma cell lines. The mRNA expression levels and MYCN gene copy number in five neuroblastoma cell lines were determined by quantitative polymerase chain reaction. In addition, variations in potential target gene expression and MYCN gene copy number between pre‑ and post‑c‑myb RNAi treatment groups in MYCN‑amplified Kelly, IMR32, SIMA and MHH‑NB‑11 cell lines, normalized to those of non‑MYCN‑amplified SH‑SY5Y, were examined. To determine the associations between gene expression levels and chromosomal aberrations, MYCN amplification and 1p36 alterations in interphases/metaphases were analyzed using fluorescence in situ hybridization. Statistical analyses revealed correlations between 1p36 alterations and the expression of c‑myb, MYB proto‑oncogene like 2 (B‑myb) and cyclin dependent kinase inhibitor 1A (p21). Additionally, the results of the present study also demonstrated that c‑myb may be associated with E2F1 and L3MBTL1 histone methyl‑lysine binding protein (L3MBTL1) expression, and that E2F1 may contribute to MYCN, B‑myb, p21 and chromatin licensing and DNA replication factor 1 (hCdt1) expression, but to the repression of geminin (GMNN). On c‑myb RNAi treatment, L3MBTL1 expression was silenced, while GMNN was upregulated, indicating G2/M arrest. In addition, MYCN gene copy number increased following treatment with c‑myb RNAi. Notably, the present study also reported a 43.545% sequence identity between upstream of MYCN and Drosophila melanogaster amplification control element 3, suggesting that expression and/or amplification mechanisms of developmentally‑regulated genes may be evolutionarily conserved. In conclusion, c‑myb may be associated with regulating MYCN expression and amplification. c‑myb, B‑myb and p21 may also serve a role against chromosome 1p aberrations. Together, it was concluded that MYCN gene is amplified during S phase, potentially via a replication‑based mechanism.
Keywords: neuroblastoma; MYCN proto‑oncogene bHLH transcription factor amplification; 1p36 deletion; c-MYB proto‑oncogene transcription factor; E2F transcription factor 1; L3MBTL1 histone methyl‑lysine binding protein; geminin DNA replication inhibitor; MYB proto‑oncogene like 2; p21/cyclin dependent kinase inhibitor 1A.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

