Cabazitaxel liposomes with aptamer modification enhance tumor‑targeting efficacy in nude mice
- PMID: 30483775
- PMCID: PMC6297770
- DOI: 10.3892/mmr.2018.9689
Cabazitaxel liposomes with aptamer modification enhance tumor‑targeting efficacy in nude mice
Abstract
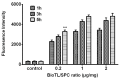
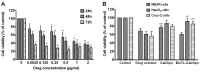
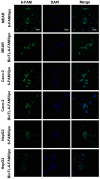
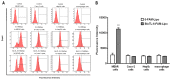
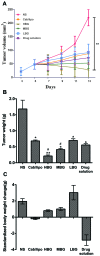
The present study investigated the feasibility of improving the tumor‑targeting efficacy and decreasing the toxicity of liposomal cabazitaxel (Cab) with aptamer modification. The process involved preparing aptamer (TLS1c)‑modified liposomes and studying the behavior of the liposomes in vitro and in vivo. TLS1c as an aptamer, which has high specificity for BNL 1ME A.7R.1 (MEAR) cells, was conjugated with Cab liposomes (Cab/lipo) to enhance MEAR tumor tissue targeting. Confocal laser scanning microscopy and flow cytometry analyses demonstrated that the fluorescence of the liposomes modified with the aptamer was notably stronger compared with that of the unmodified liposomes. Furthermore, the biodistribution data of the modified liposomes tested in tumor‑bearing mice revealed high specificity of biotinylated TLS1c‑modified Cab/lipo (BioTL‑Cab/lipo) for tumor tissues. Furthermore, the modified liposomes demonstrated decreased cytotoxicity and simultaneously retained potent inhibition against tumor growth. It is likely that the specific binding of the aptamer (TLS1c) to the targeted cells (MEAR) facilitates the binding of the liposomes to the targeted cells. Therefore, BioTL‑Cab/lipo may be considered as a promising delivery system to improve cell targeting and reduce drug toxicity in the treatment of cancer.
Keywords: aptamers; cabazitaxel; liposomes; tumor-targeting; TLS1c; nude mice.
Figures

Similar articles
-
Improvement in the drug delivery and anti-tumor efficacy of PEGylated liposomal doxorubicin by targeting RNA aptamers in mice bearing breast tumor model.Colloids Surf B Biointerfaces. 2016 Mar 1;139:228-36. doi: 10.1016/j.colsurfb.2015.12.009. Epub 2015 Dec 15. Colloids Surf B Biointerfaces. 2016. PMID: 26722819
-
RNA aptamer-conjugated liposome as an efficient anticancer drug delivery vehicle targeting cancer cells in vivo.J Control Release. 2014 Dec 28;196:234-42. doi: 10.1016/j.jconrel.2014.10.018. Epub 2014 Oct 24. J Control Release. 2014. PMID: 25450401
-
ATB0,+ transporter-mediated targeting delivery to human lung cancer cells via aspartate-modified docetaxel-loading stealth liposomes.Biomater Sci. 2017 Jan 31;5(2):295-304. doi: 10.1039/c6bm00788k. Biomater Sci. 2017. PMID: 27991616
-
Aptamer-functionalized liposomes for targeted cancer therapy.Cancer Lett. 2019 Apr 28;448:144-154. doi: 10.1016/j.canlet.2019.01.045. Epub 2019 Feb 11. Cancer Lett. 2019. PMID: 30763718 Review.
-
Small molecule therapeutics for receptor-mediated targeting through liposomes in breast cancer treatment: A comprehensive review.Bioorg Chem. 2025 Jun 15;160:108442. doi: 10.1016/j.bioorg.2025.108442. Epub 2025 Apr 5. Bioorg Chem. 2025. PMID: 40199009 Review.
Cited by
-
Aptamers for the Delivery of Plant-Based Compounds: A Review.Pharmaceutics. 2024 Apr 14;16(4):541. doi: 10.3390/pharmaceutics16040541. Pharmaceutics. 2024. PMID: 38675202 Free PMC article. Review.
-
A Biomimetic DNA-Based Membrane Gate for Protein-Controlled Transport of Cytotoxic Drugs.Angew Chem Int Ed Engl. 2021 Jan 25;60(4):1903-1908. doi: 10.1002/anie.202011583. Epub 2020 Nov 24. Angew Chem Int Ed Engl. 2021. PMID: 33231913 Free PMC article.
-
The Wnt non-canonical signaling modulates cabazitaxel sensitivity in prostate cancer cells.PLoS One. 2020 Jun 2;15(6):e0234078. doi: 10.1371/journal.pone.0234078. eCollection 2020. PLoS One. 2020. PMID: 32484838 Free PMC article.
-
On the Choice of the Extracellular Vesicles for Therapeutic Purposes.Int J Mol Sci. 2019 Jan 9;20(2):236. doi: 10.3390/ijms20020236. Int J Mol Sci. 2019. PMID: 30634425 Free PMC article. Review.
-
A Biomimetic DNA-Based Membrane Gate for Protein-Controlled Transport of Cytotoxic Drugs.Angew Chem Weinheim Bergstr Ger. 2021 Jan 25;133(4):1931-1936. doi: 10.1002/ange.202011583. Epub 2020 Nov 24. Angew Chem Weinheim Bergstr Ger. 2021. PMID: 38504763 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

