Function of BRD4 in the pathogenesis of high glucose‑induced cardiac hypertrophy
- PMID: 30483785
- PMCID: PMC6297744
- DOI: 10.3892/mmr.2018.9681
Function of BRD4 in the pathogenesis of high glucose‑induced cardiac hypertrophy
Abstract
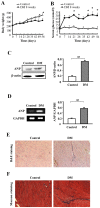
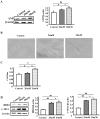
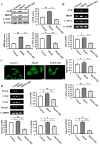
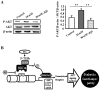
Diabetic cardiomyopathy is one of the major complications of diabetes, and due to the increasing number of patients with diabetes it is a growing concern. Diabetes‑induced cardiomyopathy has a complex pathogenesis and histone deacetylase‑mediated epigenetic processes are of prominent importance. The olfactory bromodomain‑containing protein 4 (BRD4) is a protein that recognizes and binds acetylated lysine. It has been reported that the high expression of BRD4 is involved in the process of cardiac hypertrophy. The aim of the present study was to investigate the function of BRD4 in the process of high glucose (HG)‑induced cardiac hypertrophy, and to clarify whether epigenetic regulation involving BRD4 is an important mechanism. It was revealed that BRD4 expression levels were increased in H9C2 cells following 48 h of HG stimulation. This result was also observed in a diabetic rat model. Furthermore, HG stimulation resulted in the upregulation of the myocardial hypertrophy marker, atrial natriuretic peptide, the cytoskeletal protein α‑actin and fibrosis‑associated genes including transforming growth factor‑β, SMAD family member 3, connective tissue growth factor and collagen, type 1, α1. However, administration of the specific BRD4 inhibitor JQ1 (250 nM) for 48 h reversed this phenomenon. Furthermore, protein kinase B (AKT) phosphorylation was activated by HG stimulation and suppressed by JQ1. In conclusion, BRD4 serves an important role in the pathogenesis of HG‑induced cardiomyocyte hypertrophy through the AKT pathway.
Keywords: bromodomain-containing protein 4; high glucose; cardiac hypertrophy; atrial natriuretic peptide; JQ1.
Figures

Similar articles
-
Tropisetron inhibits high glucose-induced calcineurin/NFAT hypertrophic pathway in H9c2 myocardial cells.J Pharm Pharmacol. 2016 Apr;68(4):485-93. doi: 10.1111/jphp.12522. Epub 2016 Mar 4. J Pharm Pharmacol. 2016. PMID: 26945895
-
Differential Activation of P-TEFb Complexes in the Development of Cardiomyocyte Hypertrophy following Activation of Distinct G Protein-Coupled Receptors.Mol Cell Biol. 2020 Jun 29;40(14):e00048-20. doi: 10.1128/MCB.00048-20. Print 2020 Jun 29. Mol Cell Biol. 2020. PMID: 32341082 Free PMC article.
-
BRD4 inhibition by JQ1 prevents high-fat diet-induced diabetic cardiomyopathy by activating PINK1/Parkin-mediated mitophagy in vivo.J Mol Cell Cardiol. 2020 Dec;149:1-14. doi: 10.1016/j.yjmcc.2020.09.003. Epub 2020 Sep 15. J Mol Cell Cardiol. 2020. PMID: 32941882 Free PMC article.
-
Bromodomain-containing protein 4 and its role in cardiovascular diseases.J Cell Physiol. 2021 Jul;236(7):4829-4840. doi: 10.1002/jcp.30225. Epub 2020 Dec 20. J Cell Physiol. 2021. PMID: 33345363 Review.
-
BET Protein-Mediated Transcriptional Regulation in Heart Failure.Int J Mol Sci. 2021 Jun 4;22(11):6059. doi: 10.3390/ijms22116059. Int J Mol Sci. 2021. PMID: 34199719 Free PMC article. Review.
Cited by
-
A New Family of Fused Azolo[1,5-a]pteridines and Azolo[5,1-b]purines.ACS Omega. 2020 Jul 14;5(29):18226-18233. doi: 10.1021/acsomega.0c01849. eCollection 2020 Jul 28. ACS Omega. 2020. PMID: 32743198 Free PMC article.
-
Mesenchymal Stem Cell Therapy in Diabetic Cardiomyopathy.Cells. 2022 Jan 11;11(2):240. doi: 10.3390/cells11020240. Cells. 2022. PMID: 35053356 Free PMC article. Review.
-
Research Progress on Epigenetics of Diabetic Cardiomyopathy in Type 2 Diabetes.Front Cell Dev Biol. 2021 Dec 24;9:777258. doi: 10.3389/fcell.2021.777258. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 35004678 Free PMC article. Review.
-
Autophagy enhanced by curcumin ameliorates inflammation in atherogenesis via the TFEB-P300-BRD4 axis.Acta Pharm Sin B. 2022 May;12(5):2280-2299. doi: 10.1016/j.apsb.2021.12.014. Epub 2021 Dec 29. Acta Pharm Sin B. 2022. PMID: 35646539 Free PMC article.
-
BRD2 protects the rat H9C2 cardiomyocytes from hypoxia‑reoxygenation injury by targeting Nrf2/HO‑1 signaling pathway.Exp Ther Med. 2023 Oct 4;26(5):542. doi: 10.3892/etm.2023.12241. eCollection 2023 Nov. Exp Ther Med. 2023. PMID: 37869639 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

