Lysine demethylase 2A promotes the progression of ovarian cancer by regulating the PI3K pathway and reversing epithelial‑mesenchymal transition
- PMID: 30483796
- PMCID: PMC6313075
- DOI: 10.3892/or.2018.6888
Lysine demethylase 2A promotes the progression of ovarian cancer by regulating the PI3K pathway and reversing epithelial‑mesenchymal transition
Expression of concern in
-
[Expression of Concern] Lysine demethylase 2A promotes the progression of ovarian cancer by regulating the PI3K pathway and reversing epithelial‑mesenchymal transition.Oncol Rep. 2025 Nov;54(5):138. doi: 10.3892/or.2025.8971. Epub 2025 Aug 14. Oncol Rep. 2025. PMID: 40808336
Abstract
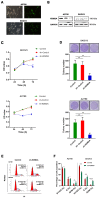
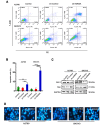
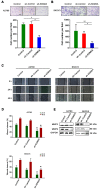
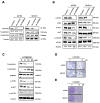
Metastasis is the most common cause of death in ovarian cancer patients but remains largely untreated. Epithelial‑mesenchymal transition (EMT) is critical for the conversion of early‑stage ovarian tumors into metastatic malignancies. Thus, investigating the signaling pathways promoting EMT may identify potential targets for the treatment of metastatic ovarian cancer. Lysine demethylase 2A (KDM2A), also known as FBXL11 and JHDM1A, is a histone H3 lysine 36 (H3K36) demethylase that regulates EMT and the metastasis of ovarian cancer. However, the function and underlying mechanisms of EMT suppression in ovarian cancer have not been thoroughly elucidated to date. In the present study, we used Gene Expression Omnibus (GEO) databases to determine that KDM2A is significantly upregulated in human ovarian cancers. KDM2A expression was assessed by immunohistochemistry of epithelial ovarian cancer (EOC) borderline ovarian tumors and normal ovary tissues. Seven fresh EOC tissues and 3 fresh normal ovary tissues were collected for western blot analysis. Kaplan‑Meier survival curves were constructed to identify genes related to EOC prognosis from the TCGA data portal. Stable KDM2A‑knockdown cell lines were established to study the biological functions and underlying mechanisms of KDM2A in EMT in vitro. GEO database analysis revealed that KDM2A was highly upregulated in EOC tissues; this analysis was accompanied by immunochemistry and western blot analysis using samples of human tissues. High expression of KDM2A was associated with poor survival in EOC patients. KDM2A knockdown promoted apoptosis and suppressed the proliferation, migration and invasion of tumor cells in vitro. EMT and the PI3K/AKT/mTOR signaling pathway were suppressed in KDM2A‑silenced cells. Inactivation of the PI3K/AKT/mTOR signaling pathway in A2780 cells induced EMT inhibition. Our data revealed that KDM2A functions as a tumor oncogene, and the downregulation of KDM2A expression regulates EMT and EOC progression, providing a valuable prognostic marker and potential target for the treatment of EOC patients.
Figures

Similar articles
-
TRIP13-induced NUSAP1 upregulation promotes CcRCC progression through EMT and PI3K/AKT/mTOR pathway.J Transl Med. 2025 Aug 11;23(1):890. doi: 10.1186/s12967-025-06761-3. J Transl Med. 2025. PMID: 40790482 Free PMC article.
-
IGF2BP1 promotes the progression of head and neck squamous cell carcinoma by activating PI3K/AKT/mTOR signaling pathway and inducing epithelial-mesenchymal transition.World J Surg Oncol. 2025 Jul 14;23(1):277. doi: 10.1186/s12957-025-03929-5. World J Surg Oncol. 2025. PMID: 40660234 Free PMC article.
-
Upregulated SAE1 Drives Tumorigenesis and Is Associated with Poor Clinical Outcomes in Breast Cancer.Breast J. 2024 Jun 30;2024:2981722. doi: 10.1155/2024/2981722. eCollection 2024. Breast J. 2024. PMID: 39742381 Free PMC article.
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article.
-
Pegylated liposomal doxorubicin for relapsed epithelial ovarian cancer.Cochrane Database Syst Rev. 2013 Jul 9;2013(7):CD006910. doi: 10.1002/14651858.CD006910.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2023 Jul 5;7:CD006910. doi: 10.1002/14651858.CD006910.pub3. PMID: 23835762 Free PMC article. Updated.
Cited by
-
Histone H3K36me2 demethylase KDM2A promotes bladder cancer progression through epigenetically silencing RARRES3.Cell Death Dis. 2022 Jun 13;13(6):547. doi: 10.1038/s41419-022-04983-7. Cell Death Dis. 2022. PMID: 35697678 Free PMC article.
-
Cellular senescence, rejuvenation and potential immortality.Proc Biol Sci. 2022 Mar 9;289(1970):20212434. doi: 10.1098/rspb.2021.2434. Epub 2022 Mar 2. Proc Biol Sci. 2022. PMID: 35232226 Free PMC article. Review.
-
Hepatitis B virus core protein stabilizes RANGAP1 to upregulate KDM2A and facilitate hepatocarcinogenesis.Cell Oncol (Dordr). 2024 Apr;47(2):639-655. doi: 10.1007/s13402-023-00889-4. Epub 2023 Oct 17. Cell Oncol (Dordr). 2024. PMID: 37845585
-
Epigenetic Regulation of Autophagy Beyond the Cytoplasm: A Review.Front Cell Dev Biol. 2021 Jun 14;9:675599. doi: 10.3389/fcell.2021.675599. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34195194 Free PMC article. Review.
-
Structure-Based Design of Selective Fat Mass and Obesity Associated Protein (FTO) Inhibitors.J Med Chem. 2021 Nov 25;64(22):16609-16625. doi: 10.1021/acs.jmedchem.1c01204. Epub 2021 Nov 11. J Med Chem. 2021. PMID: 34762429 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

