Post-stroke inflammation-target or tool for therapy?
- PMID: 30483945
- PMCID: PMC6482288
- DOI: 10.1007/s00401-018-1930-z
Post-stroke inflammation-target or tool for therapy?
Abstract
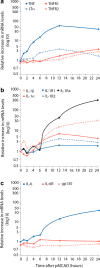
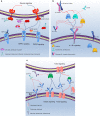
Inflammation is currently considered a prime target for the development of new stroke therapies. In the acute phase of ischemic stroke, microglia are activated and then circulating immune cells invade the peri-infarct and infarct core. Resident and infiltrating cells together orchestrate the post-stroke inflammatory response, communicating with each other and the ischemic neurons, through soluble and membrane-bound signaling molecules, including cytokines. Inflammation can be both detrimental and beneficial at particular stages after a stroke. While it can contribute to expansion of the infarct, it is also responsible for infarct resolution, and influences remodeling and repair. Several pre-clinical and clinical proof-of-concept studies have suggested the effectiveness of pharmacological interventions that target inflammation post-stroke. Experimental evidence shows that targeting certain inflammatory cytokines, such as tumor necrosis factor, interleukin (IL)-1, IL-6, and IL-10, holds promise. However, as these cytokines possess non-redundant protective and immunoregulatory functions, their neutralization or augmentation carries a risk of unwanted side effects, and clinical translation is, therefore, challenging. This review summarizes the cell biology of the post-stroke inflammatory response and discusses pharmacological interventions targeting inflammation in the acute phase after a stroke that may be used alone or in combination with recanalization therapies. Development of next-generation immune therapies should ideally aim at selectively neutralizing pathogenic immune signaling, enhancing tissue preservation, promoting neurological recovery and leaving normal function intact.
Keywords: Cytokines; Drugs; Immune therapy; Ischemia; Neuroprotection.
Figures

References
-
- Ali C, Nicole O, Docagne F, Lesne S, MacKenzie ET, Nouvelot A, et al. Ischemia-induced interleukin-6 as a potential endogenous neuroprotective cytokine against NMDA receptor-mediated excitotoxicity in the brain. J Cereb Blood Flow Metab. 2000;20:956–966. doi: 10.1097/00004647-200006000-00008. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

