Fibrous dysplasia for radiologists: beyond ground glass bone matrix
- PMID: 30484079
- PMCID: PMC6269335
- DOI: 10.1007/s13244-018-0666-6
Fibrous dysplasia for radiologists: beyond ground glass bone matrix
Abstract
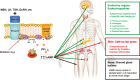
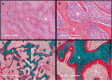
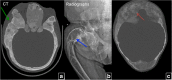
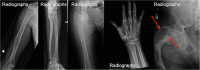
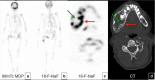
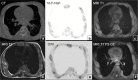
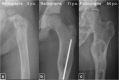
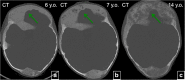
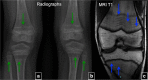
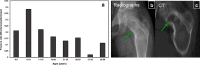
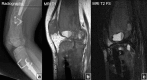
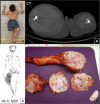
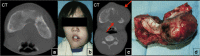
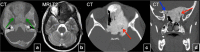
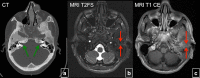
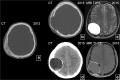
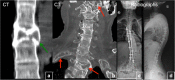
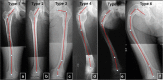
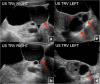
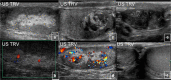
Fibrous dysplasia (FD) is a congenital disorder arising from sporadic mutation of the α-subunit of the Gs stimulatory protein. Osseous changes are characterised by the replacement and distortion of normal bone with poorly organised, structurally unsound, fibrous tissue. The disease process may be localised to a single or multiple bones. In McCune-Albright syndrome (MAS), fibrous dysplasia is associated with hyperfunction of endocrine organs and overproduction of melanin in the skin, while Mazabraud syndrome FD is associated with intramuscular myxomas. In radiology, FD is very often automatically associated with the term "ground glass matrix". However, FD is a complex disease, and knowledge of its unique pathogenesis and course are crucial to understanding imaging findings and potential complications. This article aims to not only summarise the spectrum of radiological findings of osseous and extra-osseous abnormalities associated with FD but also to highlight the pathological base of the disease evolution, corresponding imaging changes and complications based on the disease distribution. We also have provided current recommendations for clinical management and follow-up of patients with FD. TEACHING POINTS: • FD is often a part of complex disease, involving not only bone but also multiple other organs. • FD lesions are characterised by age-related histological, radiographical and clinical transformations. • Radiologists play a crucial role in the identification of osseous complications associated with FD. • The craniofacial form of the disease is the most common type of FD and the most difficult form to manage. • Patients with McCune-Albright syndrome may have different extra-skeletal abnormalities, which often require follow-up.
Keywords: Fibrous dysplasia; Ground glass bone matrix; Mazabraud’s syndrome; McCune-Albright syndrome; Skeletal radiology.
Figures

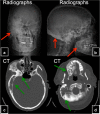
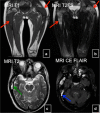
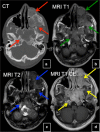
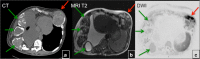
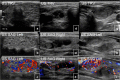
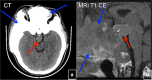
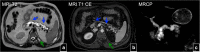
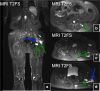
References
-
- Kozlowski K, Beighton P. Gamut index of skeletal dysplasias: an aid to radiodiagnosis. London: Springer; 2012.
Publication types
LinkOut - more resources
Full Text Sources

