Biophysical Analyses for Probing Glycan-Protein Interactions
- PMID: 30484247
- PMCID: PMC7153041
- DOI: 10.1007/978-981-13-2158-0_7
Biophysical Analyses for Probing Glycan-Protein Interactions
Abstract
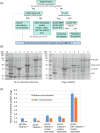
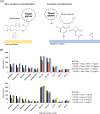
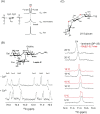
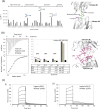
Glycan-protein interactions occur at many physiological events, and the analyses are of considerable importance for understanding glycan-dependent mechanisms. Biophysical approaches including 3D structural analysis are essential for revealing glycan-protein interactions at the atomic level. The inherent diversity of glycans suits them to function as identification tags, e.g., distinguish self from the nonself components of pathogens. However, the complexity of glycans and poor affinities for interaction partners limit the usefulness of conventional analyses. To cope with such troublesome glycans, a logical sequence of biophysical analyses need to be developed. In this chapter, we introduce a workflow of glycan-protein interaction analysis consisting of six steps: preparation of lectin and glycan, screening of glycan ligand, determination of binding epitope, quantitative interaction analysis, 3D structural analysis, and molecular dynamics simulation. Our increasing knowledge and understanding of lectin-glycan interactions will hopefully lead to the design of glyco-based medicines and vaccines.
Keywords: Frontal affinity chromatography; Glycan microarray; Isothermal titration calorimetry; Molecular dynamics simulation; Nuclear magnetic resonance; Surface plasmon resonance; X-ray crystallography.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

