The hydrophobic nature of a novel membrane interface regulates the enzyme activity of a voltage-sensing phosphatase
- PMID: 30484774
- PMCID: PMC6298786
- DOI: 10.7554/eLife.41653
The hydrophobic nature of a novel membrane interface regulates the enzyme activity of a voltage-sensing phosphatase
Abstract
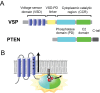
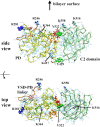
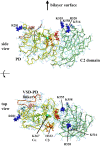
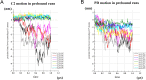
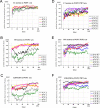
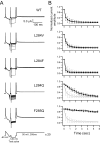
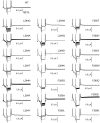
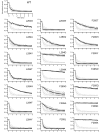
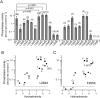
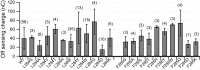
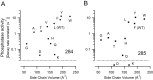
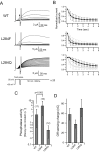
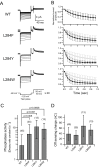
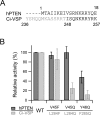
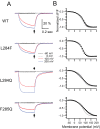
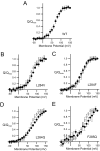
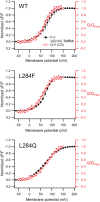
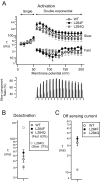
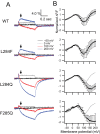
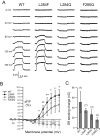
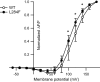
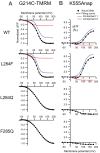
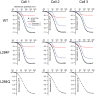
Voltage-sensing phosphatases (VSP) contain a voltage sensor domain (VSD) similar to that of voltage-gated ion channels but lack a pore-gate domain. A VSD in a VSP regulates the cytoplasmic catalytic region (CCR). However, the mechanisms by which the VSD couples to the CCR remain elusive. Here we report a membrane interface (named 'the hydrophobic spine'), which is essential for the coupling of the VSD and CCR. Our molecular dynamics simulations suggest that the hydrophobic spine of Ciona intestinalis VSP (Ci-VSP) provides a hinge-like motion for the CCR through the loose membrane association of the phosphatase domain. Electrophysiological experiments indicate that the voltage-dependent phosphatase activity of Ci-VSP depends on the hydrophobicity and presence of an aromatic ring in the hydrophobic spine. Analysis of conformational changes in the VSD and CCR suggests that the VSP has two states with distinct enzyme activities and that the second transition depends on the hydrophobic spine.
Keywords: C. intestinalis; ascidian; domain to domain coupling; membrane interaction; molecular biophysics; phosphatase; phosphoinositide; structural biology; voltage sensor.
© 2018, Kawanabe et al.
Conflict of interest statement
AK, MH, MN, KN, HN, TY, YJ, SS, AN, YO No competing interests declared
Figures







References
-
- Berendsen HJC, Postma JPM, van Gunsteren WF, DiNola A, Haak JR. Molecular dynamics with coupling to an external bath. The Journal of Chemical Physics. 1984;81:3684–3690. doi: 10.1063/1.448118. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

