Lipid transporter Spns2 promotes microglia pro-inflammatory activation in response to amyloid-beta peptide
- PMID: 30484906
- PMCID: PMC6355341
- DOI: 10.1002/glia.23558
Lipid transporter Spns2 promotes microglia pro-inflammatory activation in response to amyloid-beta peptide
Abstract
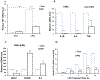
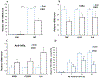
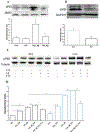
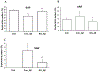
Accumulating evidence indicates that neuroinflammation contributes to the pathogenesis and exacerbation of neurodegenerative disorders, such as Alzheimer's disease (AD). Sphingosine-1-phosphate (S1P) is a pleiotropic bioactive lipid that regulates many pathophysiological processes including inflammation. We present evidence here that the spinster homolog 2 (Spns2), a S1P transporter, promotes microglia pro-inflammatory activation in vitro and in vivo. Spns2 knockout (Spns2KO) in primary cultured microglia resulted in significantly reduced levels of pro-inflammatory cytokines induced by lipopolysaccharide (LPS) and amyloid-beta peptide 1-42 oligomers (Aβ42) when compared with littermate controls. Fingolimod (FTY720), a S1P receptor 1 (S1PR1) functional antagonist and FDA approved drug for relapsing-remitting multiple sclerosis, partially blunted Aβ42-induced pro-inflammatory cytokine generation, suggesting that Spns2 promotes microglia pro-inflammatory activation through S1P-signaling. Spns2KO significantly reduced Aβ42-induced nuclear factor kappa B (NFκB) activity. S1P increased, while FTY720 dampened, Aβ42-induced NFκB activity, suggesting that Spns2 activates microglia inflammation through, at least partially, NFκB pathway. Spns2KO mouse brains showed significantly reduced Aβ42-induced microglia activation/accumulation and reduced levels of pro-inflammatory cytokines when compared with age-matched controls. More interestingly, Spns2KO ameliorated Aβ42-induced working memory deficit detected by Y-Maze. In summary, these results suggest that Spns2 promotes pro-inflammatory polarization of microglia and may play a crucial role in AD pathogenesis.
Keywords: Alzheimer's disease; Spns2; microglia; neuroinflammation; sphingosine-1-phosphate.
© 2018 Wiley Periodicals, Inc.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

