In vivo histotripsy brain treatment
- PMID: 30485186
- PMCID: PMC6925659
- DOI: 10.3171/2018.4.JNS172652
In vivo histotripsy brain treatment
Abstract
Objective: Histotripsy is an ultrasound-based treatment modality relying on the generation of targeted cavitation bubble clouds, which mechanically fractionate tissue. The purpose of the current study was to investigate the in vivo feasibility, including dosage requirements and safety, of generating well-confined destructive lesions within the porcine brain utilizing histotripsy technology.
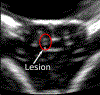
Methods: Following a craniectomy to open an acoustic window to the brain, histotripsy pulses were delivered to generate lesions in the porcine cortex. Large lesions with a major dimension of up to 1 cm were generated to demonstrate the efficacy of histotripsy lesioning in the brain. Gyrus-confined lesions were generated at different applied dosages and under ultrasound imaging guidance to ensure that they were accurately targeted and contained within individual gyri. Clinical evaluation as well as MRI and histological outcomes were assessed in the acute (≤ 6 hours) and subacute (≤ 72 hours) phases of recovery.
Results: Histotripsy was able to generate lesions with a major dimension of up to 1 cm in the cortex. Histotripsy lesions were seen to be well demarcated with sharp boundaries between treated and untreated tissues, with histological evidence of injuries extending ≤ 200 µm from their boundaries in all cases. In animals with lesions confined to the gyrus, no major hemorrhage or other complications resulting from treatment were observed. At 72 hours, MRI revealed minimal to no edema and no radiographic evidence of inflammatory changes in the perilesional area. Histological evaluation revealed the histotripsy lesions to be similar to subacute infarcts.
Conclusions: Histotripsy can be used to generate sharply defined lesions of arbitrary shapes and sizes in the swine cortex. Lesions confined to within the gyri did not lead to significant hemorrhage or edema responses at the treatment site in the acute or subacute time intervals.
Keywords: focused ultrasound; histotripsy; intracerebral hemorrhage; neurosurgery; thrombolysis.
Figures
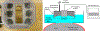






References
-
- Aronow S: The use of radio-frequency power in making lesions in the brain. J Neurosurg 17:431–438, 1960 - PubMed
-
- Clement GT, White PJ, Hynynen K: Enhanced ultrasound transmission through the human skull using shear mode conversion. J Acoust Soc Am 115:1356–1364, 2004 - PubMed
-
- Cline HE, Schenck JF, Watkins RD, Hynynen K, Jolesz FA: Magnetic resonance-guided thermal surgery. Magn Reson Med 30:98–106, 1993 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

